Abstract
Background
Psoriatic arthritis (PsA) is commonly associated with bone pathology, including entheseal new bone formation and osteolysis. On MRI, areas of active clinical involvement are represented by bone oedema and synovitis.
Aim
To assess the impact of infliximab on bone oedema in PsA as shown by MRI.
Methods
18 patients with joint swelling, psoriasis and seronegativity for rheumatoid factor received four infusions of infliximab, 3 mg/kg, in combination with methotrexate. MRI of the affected hand (12 patients) or knee joints (6 patients) was performed before and after treatment. The primary outcome was the assessment of bone oedema and synovitis at 20 weeks as shown by MRI. Secondary outcomes included the American College of Rheumatology (ACR) response criteria, psoriasis skin scores (Psoriasis Area and Severity Index (PASI)) and a quality of life measure (Psoriatic Arthritis Quality of Life (PsAQoL)).
Results
At baseline, bone oedema was seen in 50% of patients (seven hands and two knees) in 30% of scanned joints, and this improved or resolved in all cases in the hand joints (p = 0.018) and in one knee joint at 20 weeks. Synovitis was found to be reduced in 90% of cases on MRI. Likewise, a significant improvement in all clinical outcomes, including PASI (p = 0.003) and PsAQoL (p = 0.006) was seen at week 20. 65% (n = 11) of the patients achieved an ACR response, of whom 45% had ACR70 or above and 54% had ACR20 or ACR50.
Conclusions
Infliximab treatment is associated with dramatic improvements in MRI‐determined bone oedema in PsA in the short term. It remains to be determined whether infliiximib treatment is the cause for prevention of new bone formation, bone fusion or osteolysis in PsA as shown by radiography.
Psoriatic arthritis (PsA) is a common inflammatory arthritis that can cause a deforming and disabling arthropathy with a devastating effect on the quality of life.1 PsA is associated with diverse skeletal pathology such as enthesitis, synovitis, syndesmophyte formation and osteolysis, which can lead to mutilating arthritis. Active joint disease in PsA and other spondyloarthropathies (SpA) is shown as areas of bone oedema2 which are thought to represent an osteitis in the subcortical bone on MRI,3 and may occur at entheseal sites.4 Recently, tumour necrosis factor (TNF) α blockade has been a major advancement in the treatment of PsA and skin psoriasis,5,6 and MRI has shown that good clinical responses in SpA are associated with regression of enthesitis and osteitis in the axial skeleton.7,8 Likewise, MRI studies have shown a beneficial effect of anti‐TNF treatment on synovitis in PsA.9 Although there is radiographic evidence that anti‐TNF agents inhibit periarticular small joint erosion in PsA,10,11 there are no data on the effect of TNF blockade on the peripheral entheseal‐related bone manifestations of PsA. This report documents responses to a 14‐week infusion regimen of infliximab in patients with established PsA resistant to conventional disease‐modifying agents (disease‐modifying antirheumatic drugs (DMARDs)), and uses MRI to ascertain response on the bone oedema that may occur at entheseal areas in affected peripheral sites.
Patients and methods
This open‐label study was approved by the local research ethics committee, and subjects (n = 18) gave written informed consent before entry. Inclusion criteria required subjects to have at least one affected peripheral joint, history of skin psoriasis, seronegativity for rheumatoid factor and to have failed therapeutic dosages of conventional DMARDs including methotrexate. Patients had at least one clinical subtype as described by Moll and Wright,12 and were allowed to continue taking non‐steroidal anti‐inflammatory drugs and/or oral corticosteroids, provided the dose was unaltered during the study. Methotrexate was continued, but other DMARDs were stopped. Infliximab infusions (3 mg/kg in 259 ml 0.9% NaCl) were prepared by the hospital pharmacy under required aseptic conditions. The infusion regimen was planned for weeks 0, 2, 6 and 14.
Imaging
MRI of the affected hand (n = 12) or knee joints (n = 6) was performed at baseline and week 20 using a 1.5 T Gyroscan ACS‐NT whole‐body scanner (Philips Medical Systems, Best, The Netherlands).
For hand assessments, a Philips 8 cm circular surface coil was placed on the dorsum of clinically involved metacarpophalangeal (MCP) joints.2,3,4,5 T1‐weighted (T1W) and T1W post‐gadolinium‐diethylenetriamine penta‐acetic acid (Gd‐DTPA) fat‐suppressed (FS) images were acquired in the coronal and axial planes with the following parameters: coronal plane, repetition time (TR) 450 ms; echo time (TE) 20 ms; slice thickness 1.5 mm with a 0.15 mm gap; field of view (FOV) 120×100; matrix 255×256 pixels; number of signal averages (NSA) 2; axial plane, TR 420 ms; TE 20 ms; slice thickness 1.5/0.15 mm; FOV 100×50; matrix 205×256 pixels; NSA 4.
For knee assessments, a Philips quadrature receiver knee coil was used with subjects placed in the supine position for imaging. T1W and T1W post‐Gd‐DTPA FS images were acquired in the coronal, sagittal and axial planes with the following parameters: coronal, TR 450 ms; TE 20 ms; slice thickness 1.5/0.1 mm; FOV 120; NSA 2; sagittal, TR 500 ms; TE 15 ms; slice thickness 3/0.3 mm; FOV 220×65; matrix 256×256 pixels; NSA 2; and axial, TR 500 ms; TE 15 ms; slice thickness 3/0.3 mm; FOV 200×132; matrix 179×256; NSA 2. Gd‐DTPA was administered by injector pump at 0.2 ml (0.1 mmol)/kg body weight per second. Post‐Gd‐DTPA sequences began at 2.5 min after injection in the hands and at 4.5 min in the knees.
MRI‐determined bone oedema
Bone oedema was defined as an ill‐defined area in the subcortical bone with increased signal on a post‐contrast FS image. Paired scans of the hands were scored by an experienced reader who was blinded to the patients' clinical characteristics and chronology of scans, according to the Outcome Measures in Rheumatology Clinical Trials definitions.13 Diffuse areas of bone oedema that were not associated with cortical erosion and located at sites of entheseal attachments were considered to represent enthesitis and were scored using a semiquantitative scale (graded 0–3, 0 = no oedema, 1 = mild oedema, 2 = moderate, 3 = severe). The intraobserver reliability for bone oedema was moderate (wκ = 0.63, 95% CI 0.61 to 0.69).14
Bone oedema in the knee was identified in the FS images and was scored in a similar manner to that of the hand. In the absence of validated methodology, the knee joint was divided into four regions on a coronal plane: medial tibia, lateral tibia, medial femur and lateral femur; and five regions on the sagittal plane: patella, anterior femur, posterior femur, anterior tibia and posterior tibia. Bone oedema was defined as present or absent and classified as entheseal if maximal adjacent to an enthesis, using a semiquantitative scale (graded 0–3) as described previously.4 Intrarater reliability for bone oedema in the knee was good (wκ = 0.784, 95% CI 0.427 to 0.841).
Quantitative assessment of synovitis was performed on the pre‐ and post‐clinical treatment axial T1W post‐Gd‐DTPA images, which were processed using a commercially available image analysis software package Analyze (Analyze‐Direct, Lenexa, Kansas, USA). This software allowed the manual generation of regions of interest (ROIs) that delineated the enhancing synovium on consecutive slices in the MCP joints2,3,4,5 of the hand. In the knee, an ROI was positioned at the superior pole of the patella, immediately adjacent to the cartilage pannus junction, equivalent to four slices.15 The number of enhancing pixels within each ROI was calculated in each slice and converted to an area measurement. The volume of synovitis in each ROI was calculated using the formula Volsynvol = (Arsynvo × ST),16 where ST represents the sum of the slice thickness and the slice gap and Arsynvol represents the area of the synovial volume in each slice.
The analysis of paired scans was performed by one reader who was blinded to the patients' clinical characteristics. The intraobserver reliability was very good for volume of synovitis in MCP joints2,3,4,5 (ICC 0.927, 95% CI 0.890 to 0.990; n = 12) and in the suprapatellar pouch of the knee (ICC 0.995, 95% CI 0.983 to 0.998; n = 12). The response to treatment was calculated as a percentage reduction in the volume of synovitis.
Clinical assessments
Assessments performed at weeks 0, 4 and 20 included: physician and patient global assessments of disease activity (0–100 mm visual analogue scale (VAS)), patient VAS pain, Health Assessment Questionnaire and the Psoriatic Arthritis Quality of Life index.17 Swollen and tender joint counts were performed by a trained metrologist who also assessed skin involvement using the Psoriasis Area and Severity Index.18 The composite American College of Rheumatology (ACR) response criteria for rheumatoid arthritis (RA)19 was calculated, and response was defined as 20%, 50% or 70% improvement (namely ACR20, 50 and 70, respectively).
Statistical analysis
Non‐parametric tests were applied, using the Holm technique to correct for multiple testing. A p value of 0.05 was considered significant.
Results
A total of 18 patients (9:9 men:women) were recruited: median (range) age 41.5 (31–56) years, duration of PsA 14.8 (2–35) years and duration of skin psoriasis 20.3 (0–46) years. The pattern of disease was 50% polyarticular (9/18) and 39% oligoarticular (7/18); 27.7% (5/18) had a predominant distal interphalangeal joint involvement and 11% (2/18) had arthritis mutilans. In addition, past or present history of enthesitis and dactylitis was present in 89% (16/18) and 77.7% (14/18) of patients, respectively. Skin psoriasis was evident at baseline in 17 patients (median Psoriasis Area and Severity Index: 1.8). Table 1 shows a summary of the participants' baseline characteristics.
Table 1 Summary of response of the different clinical outcomes.
| Outcome measure | Baseline | Week 4 | p Value | Week 20 | p Value |
|---|---|---|---|---|---|
| VAS physician global assessment (mm) | 57 (45–90) | 27.5 (2–77) | 0.001 | 17.5 (0–61) | 0.000 |
| VAS patient disease activity (mm) | 59 (21–94) | 21 (3–68) | 0.001 | 20.5 (0–71) | 0.001 |
| VAS pain (mm) | 63.5 (22–96) | 24.5 (5–65) | 0.001 | 25.5 (0–78) | 0.003 |
| HAQ | 16.5 (6–23) | 11 (3–22) | <0.001 | 12 (0–22) | 0.000 |
| Tender joint count (68) | 14.5 (0–73) | 7 (0–65) | 0.008 | 5 (0–43) | 0.002 |
| Swollen joint count (66) | 7.5 (4–46) | 1 (0–9) | <0.001 | 0 (0–5) | 0.000 |
| EMS (min) | 120 (10–240) | 30 (0–120) | 0.002 | 20 (0–90) | 0.000 |
| CRP (mg/l) | 18.5 (2–124) | <5 (<5–10) | <0.001 | <5 (0–11) | 0.000 |
| PASI | 1.8 (0–28.1) | 0.6 (0–16.7) | 0.001 | 0 (0–13.5) | 0.003 |
| PsAQoL | 13 (<5–19) | 12 (2–19) | 0.484 | 10 (0–15) | 0.006 |
CRP, C reactive protein; EMS, early morning stiffness; HAQ, Health Assessment Questionnaire; PASI, Psoriatic Area and Severity Index; PsAQoL, Psoriatic Arthritis Quality of Life; VAS, visual analogue scale.
Values are given as median (range).
Imaging results
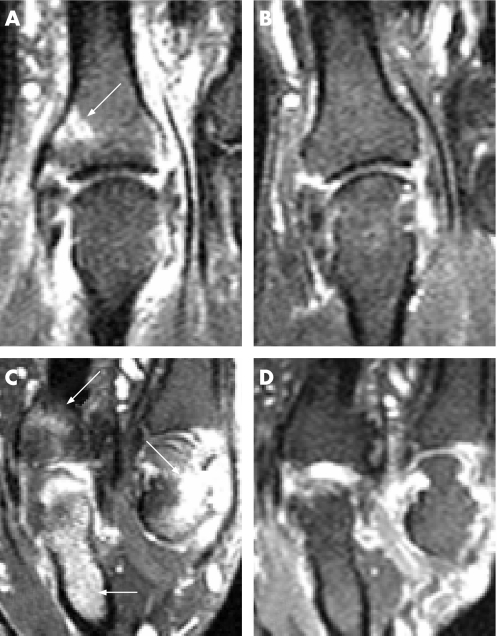
A total of 12 hand scans (n = 48 MCP joints) and 6 knee scans (n = 6 knee joints) were performed. All magnetic resonance images were suitable for assessment of bone oedema, which was seen in 50% of the patients (7 hands (n = 13 MCP joints) and 2 knees (n = 2 knee joints)) in 30% of scanned joints (n = 15/54). Of these, oedema was seen on an entheseal location, in five hand scans (corresponding to 38.4% (5/13) of MCP joints and in one knee scan (corresponding to one knee joint); however, periarticular erosions were seen at the same site in all cases. Because of the close anatomical proximity of the capsular entheses and the synovium and articular cartilage in small joints, and the diffuse nature of the MRI lesions seen in these joints, it was not always possible to state whether abnormalities were at the enthesis, and in such cases lesions were described as periarticular. Of the seven patients who had oedema at baseline in the hands, six showed complete resolution and one improved (p = 0.018; fig 1). In the knee scans, bone oedema was seen in two patients at the posterior femoral condyle: one case subchondral (associated to an erosion and unchanged on follow‐up) and the other entheseal, corresponding to the attachment of the posterior capsule (fully resolved at week 20).
Figure 1 Coronal T1‐weighted post‐gadolinium‐diethylenetriamine penta‐acetic acid fat‐suppressed images of the second (A,B) and fourth–fifth (C,D) metacarpophalangeal (MCP) joints from two of the study patients pre–and‐post treatment with infliximab. (A) The white arrow shows entheseal bone oedema distal to the joint space and there is high signal around the second MCP joint representing active synovitis‐both of which is resolved in the post‐treatment scan (B). (C) Pretreatment scan of the second patient. There is high signal representing extensive bone oedema in the proximal phalanx of the fourth finger. There is also bone oedema distal to the joint space on the fourth MCP compatible with an entheseal location, and on the proximal fifth MCP joint there is an erosion with bone oedema (white arrows). These lesions have resolved in the post‐treatment scan (D).
Scans from 15 patients (11 hands and 4 knees) were suitable for analysis of synovitis, with the rest (1 hand and 2 knees) being discarded because of technical problems (failure of FS (n = 1), enhancement (n = 1) or insufficient slice acquisition (n = 1). A reduction in the volume of synovitis was seen in 14 patients (10 hands and 4 knees). The range of synovitis reduction in the MCP joints was 9.8–60.0% and that in the ROI in the knee 37.8–91.90%.
Clinical assessment results
A significant response was seen in all clinical outcomes in all patients by week 4, which was maintained even in week 20 (table 1). Furthermore, all patients but one had an abnormal C reactive protein at baseline (mean 35.76 mg/l) that normalised after two infusions (week 4, mean 7 mg/l). At week 4, 66.6% of the patients (n = 12) achieved an ACR response: of these, 22% (n = 4) achieved ACR20, 27% (n = 5) ACR50 and 16.6% (n = 3) ACR70. This response was maintained in week 20 in 64.7% of the patients (n = 11). Of these, 17.6% (n = 3) achieved ACR20 and ACR50, and 29.4% (n = 5) achieved ACR70. One patient discontinued after the third infusion because of deranged liver function tests which normalised after discontinuation of the drug, and another patient had a recurrent urinary tract infection which was treated with antibiotics.
Discussion
This study focuses on the impact of infliximab on the bone oedema associated with active PsA. The patients were selected on the assumption that their disease was true PsA, based on its clinical features, rather than being seronegative RA and associated features.20 Although it cannot be determined whether or not the individual bone abnormalities corresponded to entheseal lesions, our results show resolution of bone oedema at most sites regardless of location, and adds to the growing body of evidence confirming the efficacy and safety of infliximab in the treatment of PsA and skin psoriasis.
Peri‐entheseal and subchondral bone oedema is a common finding at sites of disease in SpA both in the axial and in the peripheral skeleton,4,21 although its pathogenesis is not well understood. MRI can identify areas of diffuse bone oedema or osteitis, which are clearly visible at large entheseal sites such as the knee, spine and heel.4,21,22 However, owing to the smaller structure of the hand, it is difficult to say unequivocally whether diffuse peri‐entheseal bone oedema represents an enthesitis, a periarticular erosion or both.23 Indeed, in early RA, small joint erosions typically occur adjacent to collateral ligaments.14 Hence we thought it was not possible to differentiate between synovial‐ or entheseal‐based disease in the hand, and termed all bone lesions as periarticular. Furthermore, we used the current Outcome Measures in Rheumatology Clinical Trials definitions, developed for RA, which apply to lesions within 1 cm from the joint margin in the MCP joints. Although most of the lesions seen in our patients were clearly away from this margin, they were always associated with erosions, making it impossible to the human eye to establish its origin, although they were always associated with diffuse and extensive oedema, a finding noted in other sites of disease in SpA but not in RA. Higher resolution imaging techniques applied to early disease should help elucidate the pathogenic meaning of these lesions.
Bone is pivotal for the expression of PsA, and bone‐related changes including syndesmophytes and mutilating arthritis contribute significantly to the morbidity and loss of function associated with PsA. Ritchlin et al24 have suggested the role of activated osteoclasts in PsA, raising the possibility that this is a disorder of bone remodelling. Recent short‐term radiographic studies of the hand show that anti‐TNF suppresses periarticular erosion formation in patients with MCP‐related disease.10,11 However, this is the first study to focus on bone oedema in PsA and to show that oedema seen on MRI improves significantly after anti‐TNF treatment. This is an important observation, given the recent demonstration that anti‐TNF suppression of MRI‐determined spinal enthesitis and osteitis is associated with subsequent inhibition of entheseal‐related disease progression shown on radiography.25 Nevertheless, these results should be viewed in the light of the limitations of this study—namely, the small numbers studied and the fact that the trial was conducted in an open‐label fashion. Further studies involving larger number of patients are needed to determine whether in fact anti‐TNF prevents radiographic bone‐related changes in PsA.
In conclusion, our results are in accordance with previous reports of the efficacy of infliximab in the treatment of clinical and pathogenic manifestations of disease in PsA. Whether regression of MRI‐determined bone oedema in acute PsA could translate into disease modification will be confirmed in larger cohorts using more sensitive imaging methods.
Acknowledgements
We thank Sisters Claire Brown, Louise Cunningham and Ruth Thorpe for their help in coordinating this study; Drs Sheila Doherty and Michael Martin for patient referral; Drs Adam Greenstein, Steve Jarrett and Nichol Barkham for infusion supervision, and Elizabeth Hensor and Anne‐Maree Keenan for statistical advice. Infliximab was provided by Schering‐Plough (UK), who had no role in the study design, data collection or writing of the report.
Abbreviations
ACR - American College of Rheumatology
DMARD - disease‐modifying antirheumatic drug
FOV - field of view
FS - fat suppressed
Gd‐DTPA - gadolinium‐diethylenetriamine penta‐acetic acid
MCP - metacarpophalangeal
NSA - number of signal average
PASI - Psoriasis Area and Severity Index
PsA - psoriatic arthritis
PsAQoL - Psoriatic Arthritis Quality of Life
RA - rheumatoid arthritis
ROI - region of interest
SpA - spondyloarthropathies
TE - echo time
TR - repetition time
T1W - T1‐weighted
VAS - visual analogue scale
Footnotes
Competing interests: None declared.
References
- 1.Sokoll K B, Helliwell P S. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001281842–1846. [PubMed] [Google Scholar]
- 2.Savnik A, Malmskov H, Thomsen H S, Graff L B, Nielsen H, Danneskiold‐Samsoe B.et al Magnetic resonance imaging of the wrist and finger joints in patients with inflammatory joint diseases. J Rheumatol 2001282193–2200. [PubMed] [Google Scholar]
- 3.Bollow M, Fische T, Reisshauer H, Backhaus M, Hamm B, Braun J. Quantitative analysis of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis‐cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 20009135–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum 199841694–700. [DOI] [PubMed] [Google Scholar]
- 5.Antoni C E, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester G R, Schneider U.et al Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005521227–1236. [DOI] [PubMed] [Google Scholar]
- 6.Mease P J, Goffe B S, Metz J, VanderStoep A, Finck B, Burge D J. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000356385–390. [DOI] [PubMed] [Google Scholar]
- 7.Braun J, Baralakios X, Golder W, Brandt J, Rudwaleit M, Listing J.et al Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003481126–1136. [DOI] [PubMed] [Google Scholar]
- 8.Marzo‐Ortega H, McGonagle D, O'Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondyloarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum 2001442112–2117. [DOI] [PubMed] [Google Scholar]
- 9.Antoni C, Dechant C, Hanns‐Martin Lorenz P D, Wendler J, Ogilvie A, Luefti A.et al Open‐label study of infliximab treatment for psoriatic arthritis: clinical and magnetic resonance imaging measurements of reduction of inflammation. Arthritis Care Res 200247506–512. [DOI] [PubMed] [Google Scholar]
- 10.Mease P J, Kivitz A J, Burch F X, Siegel E L, Cohen S B, Ory P.et al Etanercept treatment of psoriatic arthritis. Safety, efficacy, and effect on disease progression. Arthritis Rheum 2004502264–2272. [DOI] [PubMed] [Google Scholar]
- 11.Mease P J, Gladman D D, Ritchlin C T, Ruderman E M, Steinfeld S D, Choy E H S.et al Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis. Results of a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 20053279–3289. [DOI] [PubMed]
- 12.Moll J M, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973355–78. [DOI] [PubMed] [Google Scholar]
- 13.Ostegaard M, Edmonds J, McQueen F, Peterfy C, Lasser M, Ejbjerg B.et al An introduction to the EULAR‐OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 200564(Suppl I)i3–i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown A K, Conaghan P G, Quinn M A, Karim Z, Wakefield R J, Hensor E M A.et al The reliability of magnetic resonance imaging in the assessment of low disease activity rheumatoid arthritis. Rheumatology 200645S231 [Google Scholar]
- 15.Rhodes L A, Grainger A J, Keenan A M, Thomas C, Emery P, Conaghan P G. The validation of simple scoring methods for evaluating compartment‐specific synovitis detected by MRI in knee osteoarthritis. Rheumatology 2005441569–1573. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes L A, Tan A L, Tanner S F, Radjenovic A, Hensor E M A, Reece R.et al Regional variation and differential response to therapy for knee synovitis adjacent to the cartilage‐pannus junction and suprapatellar pouch in inflammatory arthritis. Arthritis Rheum 2004502428–2432. [DOI] [PubMed] [Google Scholar]
- 17.Whalley D, Doward D, Dewar A L, McKenna S P, Tennant A, Emery P.et al The development of a quality of life instrument specific to psoriatic arthritis. Qual Life Res 19998652 [Google Scholar]
- 18.Frederiksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica 1978157238–244. [DOI] [PubMed] [Google Scholar]
- 19.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al The American College of Rheumatology preliminary definitions of improvement in rheumatoid arthritis. Arthritis Rheum 1995381–9. [DOI] [PubMed] [Google Scholar]
- 20.McGonagle D, Conaghan P G, Emery P. Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum 1999421080–1086. [DOI] [PubMed] [Google Scholar]
- 21.Braun J, Bollow M, Eggens U, Konig H, Distler A, Sieper J. Use of dynamic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondyloarthropathy patients. Arthritis Rheum 1994371039–1045. [DOI] [PubMed] [Google Scholar]
- 22.McGonagle D, Marzo‐Ortega H, O'Connor P, Gibbon W, Pease C, Reece R.et al The role of biomechanical factors and HLA‐B27 in magnetic resonance imaging‐determined bone changes in plantar fascia enthesopathy. Arthritis Rheum 200246489–493. [DOI] [PubMed] [Google Scholar]
- 23.Jevtic V, Watt I, Rozman B, Kos‐Golja M, Demsar F, Jahr O. Distinctive radiological features of small hand joints in rheumatoid arthritis and seronegative spondyloarthritis demonstrated by contrast‐enhanced (Gd‐DTPA) magnetic resonance imaging. Skelet Radiol 199524351–355. [DOI] [PubMed] [Google Scholar]
- 24.Ritchlin C T, Haas‐Smith S A, Li P, Hicks D G, Schartz E M. Mechanisms of TNF alpha and RANKL‐mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 2003111821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baraliakos X, Listing J, Brandt J, Rudwaleit M, Sieper J, Braun J.et al Influence on infliximab therapy on radiographic progression in patients with ankylosing spondylitis. Results after four years of treatment. Arthritis Rheum 200542 S631 [DOI] [PMC free article] [PubMed] [Google Scholar]