Abstract
Background
For invalidating symptoms in primary Sjögren's syndrome (pSS), there is still a need for easy‐to‐administer, cost‐effective and well‐tolerated systemic treatment. Leflunomide (LEF) is structurally unrelated to other immunomodulatory drugs and might be efficacious in pSS, given its characteristic immunoregulatory modes of action.
Objective
To investigate the safety and efficacy of LEF in pSS in a phase II open‐label pilot study.
Methods
15 patients with pSS with early and active disease received LEF 20 mg once daily for 24 weeks. Tolerability, safety and efficacy of LEF were evaluated every 8 weeks. Additional safety visits were performed every fortnight.
Results
Mild gastrointestinal discomfort (including diarrhoea) and hair loss were mainly reported. Five patients developed lupus‐like skin lesions on the face, arms or trunk, responding well to topical corticosteroids, nevertheless causing the withdrawal of one patient. Two patients with pre‐existing hypertension had to increase dosages of anti‐hypertensive drugs. Increased levels of alanine aminotransferase normalised after dose reduction in two patients. A decrease in general fatigue and an increase in physical functioning were observed after 24 weeks. Serum IgG levels decreased from 8 weeks onwards. Schirmer test values increased, not reaching statistical significance, whereas sialometry values did not change. In four of five repeated biopsies, the lymphocytic focus score decreased at the rate of 1 focus/4 mm2. A remarkable amelioration of leucocytoclastic vasculitis was observed in three patients.
Conclusions
Although the safety profile seems fairly acceptable, the observed indications for efficacy were modest and may be doubtful in justifying a randomised controlled trial of LEF in pSS.
Primary Sjögren's syndrome (pSS) is a chronic autoimmune disorder characterised by lymphoid infiltration and functional deterioration of exocrine glands, mainly the lacrimal and salivary glands, resulting in dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia). Other exocrine glands may also be affected. Usually, the combination of dryness with concomitant arthralgia, myalgia and fatigue even makes a benign course of the disease functionally invalidating.1 Several attempts to change the invalidating course of the disease have been made by using immunomodulating agents including methotrexate, usually generating disappointing results.1,2,3,4,5,6,7,8,9 A need remains for an easy‐to‐administer, cost‐effective and well‐tolerated treatment for SS (Sjögren's syndrome).
Focal T and B lymphocytic infiltration in the exocrine glands and B cell hyperactivity are the major autoimmune characteristics in SS. A dysbalance in T helper (Th) cells (proinflammatory Th1 vs anti‐inflammatory Th2 cells) is observed in both salivary glands and peripheral blood of patients with pSS.10,11,12
Leflunomide (LEF) is an isoxazol derivate structurally unrelated to other immunomodulatory drugs. LEF is rapidly metabolised to its active form, A77 1726. The primary mode of action is arresting the cell cycle of stimulated lymphocytes by selective inhibition of de novo pyrimidine synthesis by blocking the rate‐limiting enzyme dihydro‐orotate dehydrogenase.13 In addition, LEF suppresses B cell antibody response, inhibits activation and gene expression of nuclear factor κB,14,15 prevents the generation of Th1 cells and promotes differentiation to Th2 cells.16 LEF has been registered as disease‐modifying antirheumatic drug for treatment of rheumatoid arthritis and psoriatic arthritis. Several recent phase III studies demonstrated the efficacy and safety of LEF in rheumatoid arthritis for up to 5 years.17,18,19,20,21,22,23,24,25,26,27
Administration of LEF in patients with pSS might ameliorate constitutional symptoms and halt ongoing damage in exocrine glands, resulting in improved function. To decide whether a randomised, placebo‐controlled trial would be justified, we performed a phase II open‐label pilot study to investigate the safety and efficacy of LEF in 15 patients with pSS.
Patients and methods
Patients
Fifteen female patients fulfilling European–American Consensus Group criteria for pSS29 (including a lymphocytic focus score ⩾1 in labial salivary gland biopsy specimens) participated in this pilot study (table 1). Patients were randomly selected from our outpatient clinic of the Department of Rheumatology and Clinical Immunology, University Medical Center, Utrecht, The Netherlands, which is a tertially referral centre in an academic hospital. Inclusion criteria were early disease (defined as sicca complaints ⩽60 months; diagnosis established ⩽36 months) as well as active disease (erythrocyte sedimentation rate (ESR) ⩾20 mm/1st hour and/or serum IgG ⩾15 mg/l). Patients aged >18 years were eligible. Exclusion criteria were secondary Sjögren's syndrome, patients with hepatic or renal impairment, severe infection (including hepatitis B, C or HIV) and malignancy other than mucosa‐associated lymphoid tissue lymphoma (MALToma), significant cytopenia, concomitant heart and inflammatory bowel disease, pregnancy or breastfeeding status and inadequate mastery of the Dutch language. Women of childbearing age were required to use adequate contraception. Simultaneous use of other immunoregulatory agents was not allowed. The study was approved by the local medical ethics committee, and all patients gave written informed consent.
Table 1 Demographic and baseline characteristics of 15 female patients who received leflunomide 20 mg once daily during 24 weeks.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 57 | 43 | 28 | 35 | 26 | 27 | 57 | 22 | 66 | 42 | 39 | 64 | 37 | 53 | 60 |
| Sicca duration (years) | 2 | 4 | 1 | 0.5 | 2 | 4 | 4 | 2 | 4 | 5 | 5 | 2 | 5 | 5 | 3 |
| Interval diagnosis—start of study (years) | 1 | 1.5 | 1 | 3* | 1 | 2 | 3 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | 1 | 0.5 |
| Dry eyes | + | + | + | – | + | + | + | – | + | + | + | – | + | + | + |
| Dry mouth | + | + | + | – | + | + | + | – | + | – | + | + | + | + | + |
| Schirmer | 5.5 | 7.5 | 1.5 | 21 | 0 | 12 | 17.5 | 6.5 | 4 | 8.5 | 4 | 9 | NP | 5.5 | 2 |
| Sialometry | 1.4 | 3.7 | 0 | 0 | 0.3 | 0 | 0 | 0.4 | 0.7 | 0 | 0 | 1 | 0 | 0 | 0.2 |
| Parotid gland swelling | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| ESR (mm/1st hour) | 11 | 25 | 46 | 76 | 40 | 15 | 79 | 31 | 28 | 37 | 101 | 64 | 129 | 23 | |
| Serum IgG | 18.6 | 16.3 | 28.3 | 40.0 | 27.4 | 15.1 | 36.1 | 36.1 | 16.9 | 14.2 | 15.6 | 34.7 | 24.4 | 28.3 | 16 |
| Anti‐SS‐A and SS‐B | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + |
| LFS (foci/4 mm2) | 2 | 1 | 3 | 5 | 4 | 1 | 6 | 3 | 1 | ML | 6 | 6 | 6 | 1 | ML |
ESR, erythrocyte sedimentation rate; NP, not performed; LFS, lymphocytic focus score; ML, mucosa‐associated lymphoid tissue lymphoma.
Schimer test values are given in mm/5 min. Serum IgG levels are given in g/l. Sialometry values in ml after 10 min of collection of saliva of one parotid gland (see text for details).
*Diagnosis made on the basis of fatigue, increased ESR, anti‐La/SS‐B and anti‐Ro/SS‐A antibodies and characteristic findings on sublabial salivary gland biopsy. Sicca complaints have gradually developed.
Of 15 patients, 3 had concomitant lower leg leucocytoclastic vasculitis (patients 9, 10 and 13; table 1), none of them were treated with corticosteroids. A concomitant MALToma was diagnosed in the labial salivary gland tissue (patient 10) and in both parotid gland and labial salivary gland tissue (patient 15). These patients were staged according to a standard lymphoma protocol including chest x ray, CT scanning and crista biopsy, revealing no other location of lymphoma. Consequently, the diagnosis remained pSS‐related MALToma, resulting in a policy of watchful waiting.
The aim of our study being treatment of symptoms and signs of pSS, facing the lack of probability of deterioration of MALToma, in close collaboration with our haematology department, we believe the exposure of these patients with pSS to be justified. None of the patients used oral corticosteroids or immunomodulatory agents at study entry except for one patient who discontinued hydroxychloroquine at study entry.
Study design
At baseline, clinical (medical history and physical examination) and laboratory assessments were performed. Laboratory tests included a full blood count, chemistry, ESR, serum IgA, IgG and IgM levels, test for antinuclear, anti‐Ro/SS‐A and anti‐La/SS‐B antibodies and rheumatoid factor.
All patients completed the Multidimensional Fatigue Inventory (MFI), the Zung Self‐rating Depression Scale and the RAND (36‐Item Short Form (SF‐36)) questionnaire. The MFI is a 20‐item self‐report scale. We used the “general fatigue” dimension of this inventory as a reflection of fatigue.29 The Zung depression scale is a 20‐item self report scale, designed to cover affective, psychological and somatic features of depression.30 The RAND (SF‐36) questionnaire measures the quality of life as reflected in several aspects of general health, well‐being and daily life functioning31; we used the RAND (SF‐36) physical and mental component score to reflect physical functioning and mental well‐being.32
Moreover, patients reported general well‐being and subjective dryness of eyes and mouth on a visual analogue scale (VAS). As an indicator for ocular dryness, an unaesthesised Schirmer test was performed. To objectivate oral dryness, sialometry of one parotid gland was performed: saliva was collected from the parotid gland by placing a Lashley cup over the opening of Stensen's duct, while gentle negative pressure was applied on the outer concentric ring by means of a vacuum apparatus (20 kPa vacu‐aid, Hoek Loos, The Netherlands). After stimulation of salivation by placing a drop of 2% citric acid solution on the tongue, saliva was collected through the inner concentric ring of the Lashley cup into the collecting tube during 10 min.
After baseline screening, patients received oral LEF 20 mg once daily for 24 weeks. No loading dose was given; a possible benefit of rapidly reached steady‐state levels of A77 1726 was not considered to overweigh the risk of early withdrawal because of intolerance. Primary end points of the study were tolerability and safety of LEF; these were evaluated at weeks 8, 16 and 24 by questionnaires on side effects, physical examination (including blood pressure), full blood count and chemistry. Additional full blood counts and liver function tests as well as blood pressure measurements by our research nurses were performed at weeks 2, 4, 6, 10, 12, 14, 18, 20 and 22.
Efficacy of LEF was evaluated at weeks 8, 16 and 24 by the MFI, Zung and RAND (SF‐36) questionnaires, the sicca‐VAS, ESR, serum IgA, IgG and IgM and the Schirmer test. At closure of the study (24 weeks), sialometry as well as the labial salivary gland biopsy procedure was repeated to detect possible changes in lymphocytic focus scores (compared with biopsy during the diagnostic investigation). Biopsy specimens were reviewed by an experienced pathologist (R Goldschmeding), who was blinded to the patient's identity and the time of biopsy (at the time of diagnosis or at 24 weeks). Serum concentrations of A77 1726 were determined by high‐performance liquid chromatography at week 24.33
Response criteria
The Utrecht Sjögren's syndrome Response criteria, based on Pillemer et al34 (in which improvement in two of three disease domains (ocular, oral and laboratory) is determined), were used; ocular improvement was defined as ⩾20% improvement in patients' assessment of dry eyes by VAS or ⩾20% improvement in the Schirmer test. Oral improvement was defined as ⩾20% improvement in patients' assessment of dry mouth by VAS or ⩾20% improvement in parotid sialometry. Laboratory improvement was defined as ⩾20% improvement in ESR or serum IgG level. The same criteria with ⩾50% improvement in aforementioned parameters were also applied.
Statistical analysis
Student's t tests for paired data (for variables with normal distribution) or Wilcoxon's rank sum tests (for variables with non‐parametric distribution) were performed, when appropriate, to test for differences in safety and efficacy parameters between week 8 and baseline as well as between week 24 and baseline. For all variables, the last observation carried forward was applied if the last value was missing.
Differences in serum concentrations of A77 1726 in patients without and with ⩾20% improvement as well as without and with ⩾50% improvement (according to response criteria) were determined using the Mann–Whitney U test. Correlations between serum concentrations of A77 1726 and differences in efficacy parameters were calculated using Spearman's correlation coefficients.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the 15 participants with pSS, all having invalidating dryness and fatigue. All patients were women with a mean age of 43.7 years. The mean duration of sicca complaints was 3.2 years and the mean interval between diagnosis and start of the study was 1.3 years. Patients had active disease, with a mean ESR of 48 mm/1st hour, a mean serum IgG level of 23.6 g/l (normal upper limit 15.0 g/l) and a mean lymphocytic focus score in labial salivary gland biopsy specimens of 3.5 foci/4 mm2.
Safety of LEF and dose adjustments
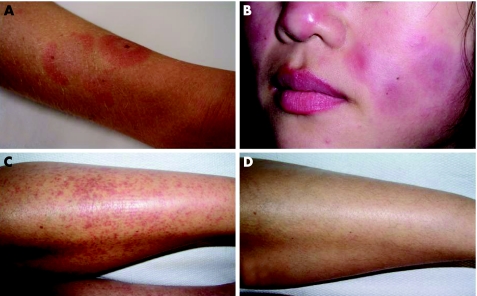
All patients except for one (patient 3) accomplished the 24‐week study period. Patient 3 withdrew at week 17 because of lupus‐like skin lesions on the face, trunk and arms (fig 1A), which had developed from approximately week 9 onwards.
Figure 1 (A) Photograph of the right lower arm of patient three who developed lupus erythematosus (LE) skin lesions on the arms, trunk and face (biopsy specimen compatible with LE) from 9 weeks onwards during treatment with leflunomide. (B) Patient eight with reactivation of LE skin lesions on face and arms, responding well to local steroid treatment. (C) Patient 13 with lower leg leucocytoclastic vasculitis at the start of the study. (D) Patient 13 at week eight; disappearance of vasculitic purpura (beyond 24 weeks).
Table 2 shows all reported and observed adverse events, including mild gastrointestinal discomfort (including diarrhoea) and hair loss, their severity decreasing during the study period. LEF was temporarily withdrawn in patients 1 and 8 because of increases in alanine aminotransferase (ALAT) levels between two and three times the upper level of normal. LEF was reintroduced at a dose of 10 mg once daily after normalisation of ALAT levels. In patient 1, no subsequent rise in ALAT levels occurred throughout the remaining study period. In patient 8, however, a subsequent repeated rise in ALAT levels again led to temporary withdrawal. Owing to an aggravation of coexisting hypertension, two patients (patients 1 and 10) had to adapt dosages of anti‐hypertensive agents. In two patients (patients 3 and 12), lupus‐like skin lesions developed de novo on the face, trunk and arms (both biopsy proven); in three patients (patients 6, 8 and 9), a reactivation of pre‐existing lupus‐like skin lesions was seen (fig 1B). In general, these skin lesions responded well to treatment with topical steroids. Mild leucopenia (white cell count between 3 and 4×109/l) developed in four patients, whereas leucopenia below 3×109/l occurred in two patients, all without infectious complications.
Table 2 All reported and/or observed adverse events in the 15 patients treated with leflunomide, indicated per individual patient.
| Patients | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Total n (%) |
| Diarrhoea | + | + | + | + | + | + | + | 7 (47) | ||||||||
| GI discomfort | + | + | + | + | + | + | 6 (40) | |||||||||
| Anorexia | + | + | 2 (13) | |||||||||||||
| Oral ulcers | + | + | 2 (13) | |||||||||||||
| Hair loss | + | + | + | + | + | + | + | 7 (47) | ||||||||
| Headache | + | + | + | + | + | 5 (33) | ||||||||||
| Fatigue/lethargy | + | + | + | 3 (20) | ||||||||||||
| Dysaesthesia | + | + | 2 (13) | |||||||||||||
| Dizziness | + | + | + | + | 4 (26) | |||||||||||
| Alcohol intolerance | + | 1 (6) | ||||||||||||||
| Weight loss >2 kg | + | + | + | + | + | 5 (33) | ||||||||||
| Dyspnoea | + | 1 (6) | ||||||||||||||
| ↑Transpiration | + | 1 (6) | ||||||||||||||
| ↑Conjunctivitis | + | + | 2 (13) | |||||||||||||
| Pharyngitis | + | + | 2 (13) | |||||||||||||
| ↓Libido | + | 1 (6) | ||||||||||||||
| Mood changes | + | 1 (6) | ||||||||||||||
| ↓Taste | + | 1 (6) | ||||||||||||||
| ALAT 1–2* ULN | + | + | 2 (13) | |||||||||||||
| ALAT >2* ULN | +* | +† | 2 (13) | |||||||||||||
| ↑Pre‐existing ↑RR | + | + | 2 (13) | |||||||||||||
| LE skin lesions | +‡§ | +¶** | +1,2 | +1,2 | + 1 | 5 (33) | ||||||||||
| Other skin lesions | + | +†† | +‡‡ | + | 3 (20) | |||||||||||
| Leucopenia 3–4×109/l | + | + | + | + | 4 (26) | |||||||||||
| Leucopenia <3×109/l | + | + | 2 (13) | |||||||||||||
| Anaemia <7.4 mmol/l | + | + | + | + | + | 5 (33) | ||||||||||
ALAT, alanine aminotransferase; GI, gastrointestinal; LE, lupus erythematosus; ULN, upper limit of normal; ↑RR, hypertension; ↓, decrease; +, present.
*Requiring temporary stop of leflunomide (LEF) treatment. After normalisation of ALAT, LEF was restarted at a dose of 10 mg OD.
†Requiring temporary stop of LEF treatment twice. After first normalisation of ALAT, LEF was restarted at a dose of 10 mg. After second normalisation, LEF 10 mg OD was restarted without subsequent increase of ALAT again.
‡Causing permanent withdrawal at 17 weeks.
§Biopsy proven.
¶Not biopsy proven.
**Reactivation of lesions that had been occurring before LEF treatment.
††Itching maculopapular rash.
‡‡Oral lesions, self‐reported, not confirmed.
Haemoglobin, white cell and thrombocyte counts decreased statistically significantly, however, without any clinical relevance (data not shown). Mean weight decreased at the rate of 1.9 kg. Weight loss >2 kg was observed in five patients (table 2).
Indications for efficacy of LEF
Table 3 gives the changes in efficacy parameters. No changes were observed in the five VAS scores of general well‐being and subjective dryness of eyes and mouth. Although a tendency towards improvement of the VAS on dry mouth was observed, statistical significance was not reached.
Table 3 Changes in efficacy parameters.
| Parameter | Mean (SD) at baseline | Mean (SD) at 8 weeks | p Value (95% CI) | Mean (SD) at 24 weeks | p Value (95% CI) |
|---|---|---|---|---|---|
| VAS general health (0–100 mm) | 58 (28) | 47 (29) | 0.137 (−3.7 to 23.9) | 51 (22) | 0.529 (−9.0 to 16.7) |
| VAS dry eyes (0–100 mm) | 53 (32) | 49 (39) | 0.614 (−12.1 to 19.8) | 60 (33) | 0.361 (−24.6 to 9.6) |
| VAS sandy feeling (0–100 mm) | 36 (39) | 45 (43) | 0.287 (−28.9 to 9.2) | 44 (39) | 0.343 (−28.5 to 10.6) |
| VAS dry mouth (0–100 mm) | 67 (31) | 65 (32) | 0.756 (−11.3 to 15.2) | 52 (35) | 0.098 (−3.0 to 32.0) |
| VAS sleep disturbance due to dryness (0–100 mm) | 43 (37) | 39 (31) | 0.304 (−4.9 to 14.7) | 38 (35) | 0.484 (−10.6 to 21.2) |
| MFI general fatigue | 17 (7)* | NP | 11 (5)* | 0.034 | |
| Zung depression score | 37 (17)* | NP | 41.5 (15.7)* | 0.726 | |
| RAND (SF‐36) physical component | 39.8 (16.0)* | NP | 43.8 (10.1)* | 0.026 | |
| RAND (SF‐36) mental component | 48.7 (23.5)* | NP | 52.1 (13.2)* | 0.790 | |
| ESR (mm/1st hour) | 48 (35) | 45 (31) | 0.346 (−3.7 to 9.8) | 42 (34) | 0.200 (−3.6 to 15.6) |
| CRP (g/l) | 8.2 (10.2) | 6.1 (4.1) | 0.287 (−2.1 to 6.4) | 6.3 (4.1) | 0.453 (−4.0 to 8.3) |
| Serum IgA (g/l) | 3.6(1.3) | 3.1 (1.2) | 0.002 (0.2 to 0.7) | 3.1 (1.1) | 0.023 (0.1 to 0.8) |
| Serum IgG (g/l) | 23.6 (8.5) | 20.4 (6.8) | 0.000 (1.7 to 4.6) | 20.2 (6.4) | 0.006 (1.2 to 5.6) |
| Serum IgM (g/l) | 1.6 (0.8) | 1.3 (0.6) | 0.004 (0.1 to 0.5) | 1.2 (0.6) | 0.005 (0.1 to 0.6) |
| RF (U/l) | 481 (664) | NP | 226 (331) | 0.045 (7 to 503) | |
| Schirmer test (mm/5 min) | 7.4 (5.9) | 9.5 (6.8) | 0.253 (−5.7 to 1.63) | 11.1 (10.6) | 0.138 (−4.6 to 0.7) |
| Sialometry (ml/15 min) | 0.8 (1.0) | NP | 0.9 (1.0) | 0.632 (−0.9 to 0.6) |
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; LFS, lymphocytic focus score; LSG, labial salivary gland; MFI, Multidimensional Fatigue Inventory; NP, not performed; RF, rheumatoid factor; SF‐36, 36‐Item Short Form; VAS, visual analogue scale.
*Medians (interquartile ranges) are given because Wilcoxon signed ranks test was used for non‐parametric distributed variables.
Four patients did not return the questionnaires at 24 weeks, consequently, the observations at 16 weeks were carried forward. A decrease in the subscore of general fatigue (MFI questionnaire) was observed (median value 11 at 24 weeks compared with 17 at baseline, p = 0.034, lower values indicating less general fatigue); in the remaining four dimensions of fatigue, however, no significant changes were found. An improvement in the physical component score of the RAND (SF‐36) questionnaire was found (median value 43.8 at 24 weeks compared with 39.8 at baseline, p = 0.026, higher scores indicating better physical functioning). No improvement was found in the RAND (SF‐36) mental component score or the Zung depression score (table 3).
ESR and CRP levels did not decrease after 8 and 24 weeks, whereas mean values of serum IgG (as a marker of disease activity) as well as of serum IgA and IgM decreased significantly from 8 weeks onwards compared with baseline. In addition, RF levels were strongly reduced after 24 weeks. The increase of the mean Schirmer test value at the rate of 3.7 mm/5 min did not reach statistical significance. Mean parotid salivary flow amounts did not increase.
Owing to refusal by six patients, labial salivary gland biopsy was repeated at 24 weeks in the remaining nine patients. In two of these patients, no representative labial salivary gland tissue was obtained. In patients 10 and 15, no relevant changes in the aspect of the MALToma were observed. In the remaining five patients in whom biopsies were repeated, labial salivary gland lymphocytic focus scores were compared with the biopsy specimens taken during diagnostic investigation: in four of them, the focus score decreased at the rate of 1 focus/4 mm2. In one patient, the focus score increased at the rate of 1 focus/4 mm2. Consequently, the mean focus score in these five patients decreased significantly from 3.6 to 3.0 foci/4 mm2 (p = 0.208).
A remarkable improvement of lower leg leucocytoclastic vasculitis was observed in patients 9, 10 and 13. Figure 1C shows vasculitic purpura on the lower legs of patient 13 at the start of the study, which improved within 8 weeks of LEF treatment (fig 1D); this improvement was sustained throughout the study period.
Serum A77 1726 concentrations and (correlations with) response criteria
The mean (SD) serum A77 1726 concentration was 38 (28) mg/l. Serum A77 1726 concentrations ranged from 14 to 116 mg/l, which is comparable with concentrations observed in patients with rheumatoid arthritis receiving the same dosage.35
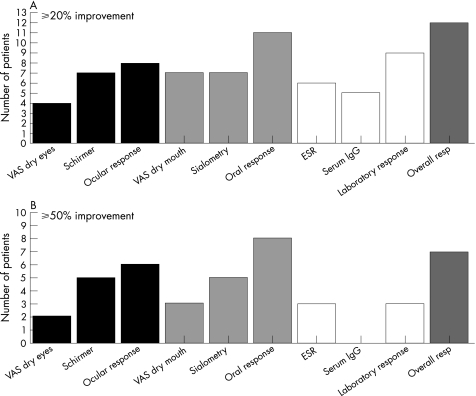
In two patients, no response criteria could be established because of missing values. Twelve patients responded to LEF according to the ⩾20% improvement criteria and seven patients even reached ⩾50% improvement (figs 2A,B). No differences were found in the A77 1726 concentrations between ⩾20% responders and non‐responders. Also, no differences were found in the A77 1726 concentrations between ⩾50% responders and non‐responders (table 4). Although a clear difference was found in A77 1726 concentrations between ⩾50% responders and non‐responders on the “laboratory domain” only (table 5), this difference did not reach significance (p = 0.145).
Figure 2 Numbers of patients fulfilling ⩾20% (upper panel) and ⩾50% (lower panel) improvement in visual analogue scale (VAS) dry eyes, Schirmer test, VAS dry mouth, sialometry, erythrocyte sedimentation rate (ESR) and serum IgG level. Numbers of patients fulfilling ocular, oral, laboratory and overall responses are depicted in the 3rd, 6th, 9th and 10th bars, respectively, in both panels (see text for details).
Table 4 Levels of A77 1726 in patients non‐responding and responding according to ⩾20% and ⩾50% criteria (see text for details).
| Response criteria | Non‐responders (n) | A 77 1726 mean (SD) | Responders (n) | A77 1726 mean (SD) | p Value of MWU test |
|---|---|---|---|---|---|
| Utrecht ⩾20% total | 2 | 49 (0.2) | 10 | 36 (30) | 0.121 |
| Utrecht ⩾20% lab only | 4 | 34 (18) | 8 | 40 (32) | 0.808 |
| Utrecht ⩾50% total | 6 | 30 (16) | 6 | 46 (36) | 0.589 |
| Utrecht ⩾50% lab only | 9 | 29 (14) | 3 | 65 (43) | 0.145 |
MWU, Mann–Whitney U test.
Of the 5 patients with representative labial salivary gland re‐biopsy, 4 (80%) responded according to ⩾50% improvement criteria. In 8 of the remaining 10 patients, response criteria could be established; 3 (38%) were ⩾50% responders.
The correlation coefficients of A77 1726 concentrations with changes in VAS values, MFI general fatigue scores, RAND (SF‐36) physical component scores, ESR, serum IgG level, Schirmer test values and sialometry values were not significant. The best correlation was found for A77 1726 concentrations with the change in Schirmer test values (r = −0.427 (p = 0.190)).
Discussion
To our knowledge, this is the first report on the treatment of LEF in pSS. In our patients, several adverse effects were observed, including those generally seen in LEF‐treated patients with rheumatoid arthritis, as well as induction or reactivation of lupus‐like skin lesions, a well‐documented phenomenon in recent literature.36,37
Owing to LEF treatment, an impressive improvement of leucocytoclastic vasculitis was observed in three patients. The MFI showed a decrease in the mean “general fatigue” subscore only; an increase in mean physical well‐being score was noted using the RAND (SF‐36).
Twelve and seven patients met ⩾20% and ⩾50% overall response criteria, respectively, which is favourable compared with numbers reported in a randomised controlled pilot study with etanercept in patients with pSS.6 Modest improvements in dry eyes and mouth were observed. However, it should be noted that a 20% or even 50% increment in Schirmer test values or parotid sialometry values may be of little clinical significance, given the fact that baseline values may be very low. The unchanging VAS values regarding dryness of eyes and mouth are in agreement with this.
The lack of correlations found between A77 1726 concentrations and changes in efficacy parameters might be explained by the small sample size. The populations with and without labial salivary gland re‐biopsy differ in numbers meeting ⩾50% overall response criteria; consequently, the observed decrease in lymphocytic focus scores should be interpreted with caution.
The observed beneficial clinical effects mediated by LEF in our pilot study might be the result of direct inhibition of lymphocyte proliferation by LEF or the restoration of the Th cell balance. However, a direct effect of LEF on B cell activity is very well possible, given the observed strong decrease in humoral responses. In line with previous reports,38,39 we found that LEF in vitro can strongly prevent activation of, in particular, Th1 cells of patients with SS (data not shown). The direct relationships of these findings with the immunopathology in SS and with the observed beneficial clinical effects need to be further elucidated.
Recent papers describe the increase in salivary gland function in patients with pSS with residual salivary gland function treated with rituximab, a B cell‐depleting agent.40,41 This is a promising treatment, keeping in mind the observed adverse events and high costs. In our LEF‐treated patients with pSS with residual salivary gland function, no significant increment in salivary function was observed, possibly because of different methods of sialometry or only partial inhibition of B cell function by LEF.
In this pilot study, the safety profile of LEF in pSS seems fairly acceptable. However, the observed indications for efficacy were modest and may be doubtful in justifying a randomised controlled trial of LEF in pSS.
Acknowledgements
This study was financially supported by Sanofi‐Aventis, The Netherlands and the Dutch Association for Sjögren patients (Nationale Vereniging Sjögren Patiënten, Bunnik, the Netherlands). We thank Etienne Blaas and Suzanne Bakker‐ van Wijk for excellent nursing assistance.
Abbreviations
ALAT - alanine aminotransferase
LEF - leflunomide
MALToma - mucosa‐associated lymphoid tissue lymphoma
MFI - Multidimensional Fatigue Inventory
pSS - primary Sjögren's syndrome
SF‐36 - 36‐Item Short Form
SS - Sjögren's syndrome
Th - T helper
VAS - visual analogue scale
Footnotes
Competing interests: None declared.
References
- 1.Valtysdottir S T, Gudbjornsson B, Hallgren R, Hetta J. pp. 597–600. [PubMed]
- 2.Kruize A A, Hene R J, Kallenberg C G, van Bijsterveld O P, van der Heide A, Kater L et a l. pp. 360–364. [DOI] [PMC free article] [PubMed]
- 3.Kruize A A, Hene R J, Vianen M E, Lafeber F P, Bijlsma J W. Systemic treatment of patients with Sjögren's syndrome: not with sulfasalazine [thesis]. Utrecht: Utrecht University, 1997;81–90,
- 4.Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E.et alInefficacy of infliximab in primary Sjogren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjogren's Syndrome (TRIPSS). Arthritis Rheum 2004;501270–1276. [DOI] [PubMed]
- 5.Price E J, Rigby S P, Clancy U, Venables P J. pp. 896–899. [PubMed]
- 6.Sankar V, Brennan M T, Kok M R, Leakan R A, Smith J A, Manny J.et alEtanercept in Sjogren's syndrome: a twelve‐week randomized, double‐blind, placebo‐controlled pilot clinical trial. Arthritis Rheum 2004;502240–2245. [DOI] [PubMed]
- 7.Shiozawa S, Tanaka Y, Shiozawa K. pp. 255–262. [DOI] [PubMed]
- 8.Skopouli F N, Jagiello P, Tsifetaki N, Moutsopoulos H M. pp. 555–558. [PubMed]
- 9.Zandbelt M M, de Wilde P, van Damme P, Hoyng C B, van de Putte L, van den Hoogen F. pp. 96–101. [PubMed]
- 10.Fox R I, Kang H I, Ando D, Abrams J, Pisa E. pp. 5532–5539. [PubMed]
- 11.Hagiwara E, Pando J, Ishigatsubo Y, Klinman D M. pp. 89–93. [PubMed]
- 12.van Woerkom J M, Kruize A A, Wenting‐van Wijk M J, Knol E, Bihari I C, Jacobs J W G.et al Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjogren's syndrome compared to non‐Sjogren's sicca syndrome. Ann Rheum Dis 2005641474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox R I. pp. 20–26. [PubMed]
- 14.Manna S K, Mukhopadhyay A, Aggarwal B B. pp. 5962–5969. [DOI] [PubMed]
- 15.Siemasko K F, Chong A S, Williams J W, Bremer E G, Finnegan A. pp. 635–642. [DOI] [PubMed]
- 16.Dimitrova P, Skapenko A, Herrmann M L, Schleyerbach R, Kalden J R, Schulze‐Koops H. pp. 3392–3399. [DOI] [PubMed]
- 17.Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G.et alTreatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med 1999;1592542–2550. [DOI] [PubMed]
- 18.Cohen S, Cannon G W, Schiff M, Weaver A, Fox R, Olsen N.et alTwo‐year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis Rheum 2001;441984–1992. [DOI] [PubMed]
- 19.Emery P, Breedveld F C, Lemmel E M, Kaltwasser J P, Dawes P T, Gömör B.et alA comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000;39655–655. [DOI] [PubMed]
- 20.Kalden J R, Scott D L, Smolen J S, Schattenkirchner M, Rozman B, Williams B D.et alImproved functional ability in patients with rheumatoid arthritis—longterm treatment with leflunomide versus sulfasalazine. European Leflunomide Study Group. J Rheumatol 2001;281983–1991. [PubMed]
- 21.Scott D L, Smolen J S, Kalden J R, van de Putte L B A, Larsen A, Kvien T K.et alTreatment of active rheumatoid arthritis with leflunomide: two year follow up of a double blind, placebo controlled trial versus sulfasalazine. Ann Rheum Dis 2001;60913–913. [DOI] [PMC free article] [PubMed]
- 22.Kaltwasser J P, Nash P, Gladman D, Rosen C F, Behrens F, Jones P.et alEfficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double‐blind, randomized, placebo‐controlled clinical trial. Arthritis Rheum 2004;501939–1950. [DOI] [PubMed]
- 23.Remer C F, Weisman M H, Wallace D J. pp. 480–483. [DOI] [PubMed]
- 24.Tam L S, Li E K, Wong C K, Lam C W K, Szeto C ‐ C. pp. 601–604. [DOI] [PubMed]
- 25.Metzler C, Fink C, Lamprecht P, Gross W L, Reinhold‐Keller E. pp. 315–320. [DOI] [PubMed]
- 26.Talip F, Walker N, Khan W, Zimmermann B. pp. 868–870. [PubMed]
- 27.Cefle A. pp. 764–767. [DOI] [PubMed]
- 28.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H M, Alexander E L, Carsons S E.et alClassification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002;61554–558. [DOI] [PMC free article] [PubMed]
- 29.Smets E M, Garssen B, Bonke B, de Haes J C. pp. 315–325. [DOI] [PubMed]
- 30.Zung W W, Broadhead W E, Roth M E. pp. 337–344. [PubMed]
- 31.van der Zee K, Sanderman R, Heyink J, de Haes H. pp. 104–122. [DOI] [PubMed]
- 32.Ware J, Kosinski M, Keller S. Physical and mental health suary scales—a user's manual. Boston, MA: New England Medical Center, The Health Institute, 1994
- 33.van Roon E N, Yska J P, Raemaekers J, Jansen T L, van Wanrooy M, Brouwers J R. pp. 17–22. [DOI] [PubMed]
- 34.Pillemer S R, Brennan M T, Sankar V, Leakan R A, Smith J A, Grisius M.et alPilot clinical trial of dehydroepiandrosterone (DHEA) versus placebo for Sjogren's syndrome. Arthritis Rheum 2004;51601–604. [DOI] [PubMed]
- 35.van Roon E N, Jansen T L, van de Laar M A, Janssen M, Yska J P, Keuper R.et alTherapeutic drug monitoring of A77 1726, the active metabolite of leflunomide: serum concentrations predict response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64569–574. [DOI] [PMC free article] [PubMed]
- 36.Gensburger D, Kawashima M, Marotte H, Kanitakis J, Miossec P. pp. 153–155. [DOI] [PMC free article] [PubMed]
- 37.Goeb V, Berthelot J M, Joly P, Mejjad O, de Quatrebarbes J, Reynaud‐Hautin C.et alLeflunomide‐induced subacute cutaneous lupus erythematosus. Rheumatology (Oxford) 2005;44823–824. [DOI] [PubMed]
- 38.Kraan M C, Smeets T J, van Loon M J, Breedveld F C, Dijkmans B A C, Tak P P. pp. 1056–1061. [DOI] [PMC free article] [PubMed]
- 39.Magari K, Miyata S, Nishigaki F, Ohkubo Y, Mutoh S. pp. 544–550. [DOI] [PubMed]
- 40.Pijpe J, van Imhoff G W, Vissink A, van der Wal J E, Kluin P M, Spijkervet F K.et alChanges in salivary gland immunohistology and function after rituximab monotherapy in a patient with Sjogren's syndrome and associated MALT lymphoma. Ann Rheum Dis 2005;64958–960. [DOI] [PMC free article] [PubMed]
- 41.Pijpe J, van Imhoff G W, Spijkervet F K, Roodenburg J L, Wolbink G J, Mansour K.et alRituximab treatment in patients with primary Sjogren's syndrome: an open‐label phase II study. Arthritis Rheum 2005;522740–2750. [DOI] [PubMed]