Abstract
Objectives
To characterise the phenotype of the putative dendritic cells strongly expressing Jak3 and STAT4, which have been previously identified in the synovial tissue of patients with active rheumatoid arthritis (RA).
Methods
Synovial biopsy specimens were obtained at arthroscopy from 30 patients with active RA (42 synovial biopsies). Immunohistological analysis was performed using monoclonal antibodies to detect dendritic cell subsets, including activation markers and cytokines relevant to dendritic cell function. Co‐localisation of cell surface markers and cytokines was assessed primarily using sequential sections, with results confirmed by dual immunohistochemistry and immunofluorescence with confocal microscopy.
Results
The dendritic cells identified in RA synovial tissue that strongly express Jak3 also strongly express STAT4 and STAT 6 and are correlated with the presence of serum rheumatoid factor. These cells are not confined to a single dendritic cell subset, with cells having phenotypes consistent with both myeloid‐ and plasmacytoid‐type dendritic cells. The activation status of these dendritic cells suggests that they are maturing or mature dendritic cells. These dendritic cells produce IL12 as well as interferon α and γ.
Conclusions
The close correlation of these dendritic cells with the presence of serum rheumatoid factor, a prognostic factor for worse disease outcome, and the strong expression by these cells of components of the Jak/STAT transcription factor pathway suggest a potential therapeutic target for the treatment of RA.
Keywords: rheumatoid arthritis, myeloid dendritic cells, plasmacytoid dendritic cells, IL12, interferon alpha, interferon gamma
Targeting dendritic cells (DCs) as a means of modulating immune responses is an exciting and rapidly developing field, with particular applications in transplant medicine, oncology and autoimmunity. In the peripheral blood, two principal DC subsets are recognised, based on CD11c and CD123 expression. Myeloid DCs (moDCs) are CD11c+ CD123lo and have a monocytoid appearance. moDCs can be further subdivided into CD14+ blood monocyte‐derived DCs, dermal DCs or interstitial DCs and Langerhan's cells. These are the “conventional” DCs and in their immature state, they are efficient at antigen uptake and processing and act as sentinels in peripheral tissues. After activation (eg, from CD40 ligand, interleukin (IL)‐1, IL6 and interferon (IFN)α or stimulation of Toll‐like receptors by microbial products or damaged tissue) they undergo maturation and migrate to secondary lymphoid tissues, where they initiate immune responses.1 Maturation is characterised by increased expression of costimulatory molecules and adhesion molecules, which are required for efficient antigen presentation to T cells.2 Mature moDCs typically produce large amounts of IL12 and IFNγ, critical in promoting Th1‐type responses3,4 as well as tumour necrosis factor α, IL1β and IL6.5
Plasmacytoid DCs (PDCs) are CD11c− CD123hi and have a plasma cell‐like morphology. moDCs and PDCs differ in their tissue distribution, cytokine production and growth requirements.2,6 PDCs differ from moDCs in several important respects. Human PDCs are CD4+, CD45RA+, CD123+, CD11c− lineage cells. They can be identified by the presence of the α‐chain of the IL3 receptor (CD123) and the expression of two specific markers, BDCA‐2 and BDCA‐4. BDCA‐2 is a C‐type lectin receptor thought to have a role in antigen capture and presentation.7 In addition, PDCs have a distinct set of chemokine and Toll‐like receptors.
Freshly isolated PDCs are inferior antigen‐presenting cells compared with moDCs. This may be explained by several factors, including the absence of cathepsin S and cathepsin D, involved in antigen processing. In addition, they have minimal expression of costimulatory molecules and low major histocompatibility complex (MHC) II expression relative to conventional DCs. Once activated, their T‐cell regulatory capacity increases, coincident with upregulation of MHC II and costimulatory molecules, but they still remain less efficient than moDCs. Under in vivo conditions, PDCs may also coordinate with conventional DCs to optimise T‐cell proliferation and differentiation.8 PDCs are the most efficient IFNα/β‐producing cells in the body; moDCs can also be activated to produce IFNα/β, although to a lesser extent than PDCs. PDCs have an important role in modulating viral disease and have been found to contribute to initiation and amplification of T‐cell activation. Another very important role of PDCs is their ability, through production of type I interferons and IL6 to generate plasma cells and the antibody response. The production of IL12 by human PDCs is more controversial.8,9
Under resting conditions, DCs exist in an “immature state” and are weak stimulators of T cells. They have few MHC class II and accessory molecules but many antigen‐capturing receptors. Immature DCs also have critical roles in the maintenance of self‐tolerance in the steady state.6 Captured antigens induce maturation of DCs. After activation, DCs travel to the lymphoid tissues where they complete their maturation and become potent antigen‐presenting cells. Recent evidence has demonstrated interchangeability between myeloid and plasmacytoid phenotype DCs. In a murine model, PDCs have been shown to convert to moDCs after viral infection,10 and Flt3‐expressing myeloid and lymphoid progenitors can give rise to PDCs and moDCs.2
DCs have long been known to have a critical role in regulating immunity, but their relevance to rheumatoid arthritis (RA) has only been recently realised.1,11 In 1994, Thomas and coworkers described mature DCs in the rheumatoid synovium.12 These initial observations were followed by numerous reports further characterising DC populations in rheumatoid synovium, synovial fluid and other forms of inflammatory arthritis. DCs in synovial tissue were shown to have phenotypical differences from those of healthy controls.11 CTLA4Ig (T lymphocyte associated antigen 4 immunoglobulin) inhibits optimal interaction between T cells and DCs by blocking the interaction between the DC costimulatory molecules CD80 and CD86 with CD28 on T cells.11,13 Evidence indicating the important role of DCs in promoting inflammation in RA has also accumulated,14 supported by the recent finding that CTLA4Ig has promising effects in phase II trials as a treatment for RA.13 PDCs and moDCs are likely to have significantly differing roles as immune modulators within the rheumatoid synovium, based on the cytokines they produce. Therefore, both moDCs and PDCs are likely to contribute to inflammation in the rheumatoid synovium in differing ways.
DC function has the potential to be modulated, either through genetic engineering15 or pharmaceutical drug treatment, including modulation of the Jak/STAT pathway and specifically Jak3.16,17 Therefore the identification of a population of DCs within RA tissue that expresses high levels of Jak3 and other components of the Jak/STAT pathway would provide a potential therapeutic target in the treatment of RA.
Transcription factors bridge the gap between cytokine–receptor interaction at the cell surface and the transcriptional effects of this interaction in the cell nucleus. There are a limited number of inducible transcription factors which seem to have a pivotal role in the regulation of inflammatory genes (eg, activator protein‐1 (AP‐1), CCAT/enhancer‐binding proteins (C/EBPs), signal transducer and activator of transcription (STATs), nuclear factor of activated T cells (NF‐AT) and nuclear factor‐kappa B (NF‐κB). The janus kinase (Jak) and STAT pathway is the signalling target of a multitude of cytokines, including IFNγ, IL2, IL4, IL6, IL7, IL10, IL12 and IL15, all of which are thought to have biologically significant roles in rheumatoid synovial inflammation.18 We have previously published a report of Jak/STAT expression in RA synovial tissue and identified a subpopulation of cells specific to seropositive RA that show dendritic morphology and stain intensely for Jak3, STAT4 and STAT6.19 Initial phenotyping of these cells demonstrated that they were CD1a+ CD22− CD68− CD3− CD55−, which was consistent with a DC phenotype. We further postulated that these may be DCs in the early stages of activation. This study aimed at characterising further this interesting population of cells in the rheumatoid synovium which strongly express Jak/STAT transcription and identifying the cytokines they produce.
Materials and methods
All patients with RA fulfilled the American College of Rheumatology criteria for RA.20 All patients gave informed consent, and the study protocol was approved by the research and ethics committee of the Repatriation General Hospital, Adelaide, South Australia. Synovial membrane samples were obtained from clinically affected knee joints (42 biopsies) of 30 patients with active RA under direct vision using a 2.7 mm mini‐arthroscope (Dyonics, Andover, MA, USA) and standard approaches, as previously described.21
Table 1 gives clinical and laboratory details of the patients studied, including age, disease duration, disease‐modifying antirheumatic drug (DMARD) use, serum levels of rheumatoid factor (RF, normal <40 U/ml)) and C‐reactive protein (CRP, normal <6 mg/l) and Jak/STAT expression in synovial tissue.
Table 1 Demographics of patients included in this study, including expression of Jak3/STAT4 in synovial tissue.
| Patient | Age | RF (U/ml) | CRP (mg/l) | DMARD use | Disease duration (months) | Jak3 | STAT4 |
|---|---|---|---|---|---|---|---|
| 1a* | 76 | 43 | 130 | Methotrexate | 3 | – | – |
| 1b | 76 | 35 | 1 | Methotrexate | 7 | – | – |
| 2a | 77 | 141 | 15 | IM gold | 3 | + | + |
| 2b | 76 | 20 | 5 | IM gold | 14 | – | – |
| 3a | 65 | 263 | 84 | IM gold/methotrexate | 9 | – | + |
| 3b | 67 | 372 | 4 | IM gold/methotrexate | 24 | – | – |
| 4a | 66 | 486 | 162 | IM gold/methotrexate | 36 | + | + |
| 4b | 67 | 94 | 19 | IM gold/methotrexate | 48 | – | – |
| 5a | 75 | 20 | 20 | Methotrexate | 36 | – | – |
| 5b | 78 | 20 | 1 | Methotrexate | 36 | – | – |
| 6a | 77 | 504 | 16 | IM gold/methotrexate | 180 | + | + |
| 6b | 78 | 145 | 9 | Methotrexate | 192 | + | + |
| 7a | 76 | 351 | 62 | IM gold | 3 | + | + |
| 7b | 78 | 22 | 4 | IM gold | 9 | – | – |
| 8a | 73 | 143 | 26 | IM gold/methotrexate | 6 | + | + |
| 8b | 75 | 20 | 1 | Methotrexate | 20 | – | – |
| 9a | 70 | 404 | 116 | IM gold/methotrexate | 12 | – | + |
| 9b | 73 | 25 | 1 | Methotrexate | 42 | – | + |
| 10a | 56 | 400 | 167 | IM gold/methotrexate | 168 | + | + |
| 10b | 57 | 36 | 12 | IM gold/methotrexate | 180 | – | – |
| 11a | 83 | 20 | 83 | Methotrexate | 180 | – | – |
| 11b | 85 | 20 | 1 | Methotrexate | 204 | – | – |
| 12a | 60 | 248 | 17 | SSZ, prednisolone | 132 | + | + |
| 12b | 59 | 499 | 110 | IM gold/methotrexate | 120 | – | – |
| 13 | 75 | 213 | 14 | Ciclosporin A | 84 | + | + |
| 14 | 59 | 531 | 35 | Methotrexate, SSZ | 216 | + | + |
| 15 | 43 | 20 | 90 | Methotrexate/SSZ | 6 | + | + |
| 16 | 74 | 101 | 5 | IM gold, methotrexate | 12 | + | + |
| 17 | 36 | 80 | 3 | Ciclosporin A, prednisolone, NSAID | 156 | + | + |
| 18 | 63 | 20 | 1 | NSAID | 12 | – | – |
| 19 | 71 | 1220 | 120 | Nil | 3 | + | + |
| 20 | 41 | 26 | 1 | Nil | 6 | – | – |
| 21 | 74 | 1552 | 9 | Nil | 24 | + | + |
| 22 | 48 | 335 | 8 | Nil | 12 | + | + |
| 23 | 84 | 20 | 90 | Methotrexate, prednisolone | 84 | – | – |
| 24 | 27 | 20 | 37 | SSZ | 2 | + | + |
| 25 | 72 | 155 | 27 | SSZ/prednisolone | 120 | + | + |
| 26 | 80 | 146 | 88 | IM gold/methotrexate | 36 | – | – |
| 27 | 77 | 568 | 61 | IM gold | 3 | + | + |
| 28 | 74 | 101 | 5 | IM gold/methotrexate | 48 | – | + |
| 29 | 58 | 20 | 7 | IM methotrexate/IM gold/Arava | 36 | – | – |
| 30 | 60 | 818 | 26 | NSAIDs only | 228 | + | + |
IM, intramuscular; NSAID, non‐steroidal anti‐inflammatory drug; SSZ, sulfasalazine.
*a, b used to denote cases where more than one biopsy specimen was taken from the same patient.
Immunohistochemistry
Cryosections (4 μm thick) were prepared on APTS (3‐ aminopropyltriethoxysilane; Sigma, St Louis, MO, USA)‐treated glass slides and fixed in ice‐cold acetone for 4 minutes. Sections were brought to room temperature, washed in phosphate‐buffered saline, and immunohistochemical labelling for Jak3, STAT4 and STAT6 (Santa Cruz Biotechnology Inc, CA, USA) was performed on all tissues using a double enhancement method, as previously published.19 To eliminate bias from run‐to‐run variability, all staining for a particular antibody was performed on all synovial tissue samples on the same day. For dual immunohistochemistry, we combined an immunoperoxidase technique with anti‐STAT4 as the primary antibody and AEC as the chromagen with an immunoalkaline phosphatase technique using antibodies against DC markers as primary antibodies (see below) and fast blue as the chromagen, as previously published.22
Expression of STAT4 was used for co‐localisation studies, using representative synovial tissue, previously shown to stain for Jak3 and STAT4, from four patients with RA. The following antibodies were used in co‐localisation studies to characterise further the potential dendritic nature of these cells (table 2).
Table 2 Epitopes that distinguish human DC types (adapted from O'Garra and Trinchieri10).
| Epitopes | Conventional or myeloid dendritic cells (DCs)* | Plasmacytoid DCs† | ||
|---|---|---|---|---|
| Langerhan's cells | Interstitial DCs | Monocyte‐derived DCs | ||
| CD1a | + | + | + | – |
| CD1d | – | + | + | Unknown |
| CD11b | – | + | + | – |
| CD11c | + | + | + | – |
| CD52 | – | – | + | Unknown |
| CD83‡ | + | + | + | + |
| e‐Cadherin§ | + | – | – | – |
| CD207, Langerin§ | + | – | – | – |
| CD208, DC‐LAMP‡ | + | + | + | – |
| CD123 | + | + | + | ++ |
| BDCA‐2, 4¶ | –/+ | Unknown | –/+ | + |
| DEC‐205 | + | + | + | ?+ |
| CMRF56 | + | Unknown | + | Unknown |
*All myeloid human DCs are class II MHCbright, CD80++/+++, CD86+++, CD83+, CD14neg, and CD11c+. Immature DCs express class II MHC, CD80, and CD86, and these increase with maturation. CD14 is lost with differentiation of moDCs from monocyte precursors. Interstitial DCs develop from CD34+ haematopoietic progenitor cells via a CD14+ intermediate.
†Freshly isolated plasmacytoid DCs express much lower levels of MHC and costimulatory molecules than their freshly isolated or immature, conventional DC counterparts.
‡Expressed after full maturation. CD83 epitope density is highest on mature moDCs.
§Immature LCs express E‐cadherin and Langerin. These markers decrease with maturation.
¶BDCA‐4 is upregulated in cultures of moDCs. BDCA‐2 is downregulated in cultures of plasmacytoid DCs.
CD1a (Serotec, Oxford, UK), an immature DC marker; CMRF56 (gift from Professor Derek Hart, Queensland Medical Institute, Queensland, Australia), an early DC activation marker; CD83 (Serotec, Oxford, UK), p55 (Dakocytomation, Botany, NSW, Australia) and DEC‐205 (Gift from Masato Kato, Mater Medical Research Institute, Queensland, Australia), mature dendritic cell markers; BDCA‐2 (Miltenyl Biotec, Gladbach, Germany) and CD123 (BD Biosciences, San Diego, USA), PDC markers. R4/23 (Dakocytomation, Botany, NSW, Australia), an interdigitating reticulum DC marker was also used.
Dual labelling immunofluorescence with confocal microscopy and sequential section staining were all tested for their effectiveness in detecting co‐labelling with DC markers. We found sequential section staining to be the most reliable method of assessing co‐localisation as technical difficulties made dual labelling difficult to interpret because the intensity of Jak3 and STAT4 staining using AEC largely overwhelmed any fast blue staining for DC markers. Therefore most of the co‐localisation was performed on sequential sections, with confirmation of results using dual immunohistochemistry and immunofluorescence with confocal microscopy.
To provide further information about the stage of activation and phenotype of these cells, expression of IFNγ (R & D Systems, MN, USA), IL12 (R & D Systems, MN, USA) and IFNα (PBL Biomedical Laboratories, NJ, USA) were assessed. IFNγ and IL12 are produced in increasingly large quantities by conventional dendritic cells as they undergo maturation, whereas IFNα and, more controversially, IL12 are produced by PDCs.
Statistical analysis
Spearman's rank correlation and Kendall's τ analysis were used for statistical analysis, and an RF >40 IU/ml was considered positive. A p value <0.05 was considered to be significant.
Results
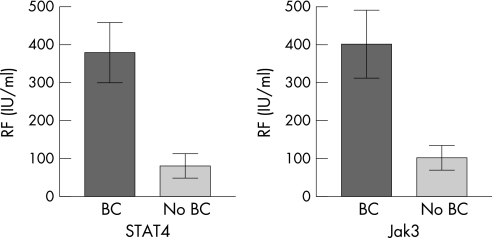
Our initial work in 10 patients with RA showed a strong positive correlation between the presence of RF in the serum and Jak3/STAT4/STAT6 bright cells, with no correlation with CRP, DMARD usage, duration of disease, age, or gender in this small cohort of patients with RA.19 These results were confirmed in a larger group of 30 patients with RA in this study as shown in fig 1. Jak3 and STAT4 bright cell expressions were moderately correlated with RF positivity (r2 = 0.552, p<0.001; r2 = 0.609 p<0.001, respectively). As shown previously, there was no significant correlation between Jak3/STAT4 expression and patient age, disease duration, CRP or DMARD use.
Figure 1 Correlation of Jak3 and STAT 4 “bright cells (BC)” with serum levels of rheumatoid factor (RF) in the patient group. Results shown are means with error bars indicating standard deviations.
As we have previously shown in a smaller patient cohort, expression of Jak3 bright cells was found to be strongly correlated with STAT4 bright cells (r2 = 0.826, p<0.0001). In a subpopulation of this patient group (n = 11 patients, 22 biopsies), STAT6 bright cells were found to be strongly correlated with Jak3 and STAT4 bright cell expression (τ = 0.790, p<0.001).
Phenotyping studies with DC markers
We have previously shown that Jak3/STAT4 bright cells do not co‐localise with CD45 (T cell marker), CD22 (B cell marker), CD68 (macrophage marker) or CD55 (lining fibroblast marker); however, they did co‐localise with CD1a, an immature DC marker.19 To further define the bright cell population, we performed a combination of sequential staining, immunofluorescence with confocal microscopy and dual immunohistochemical labelling to assess co‐localisation.
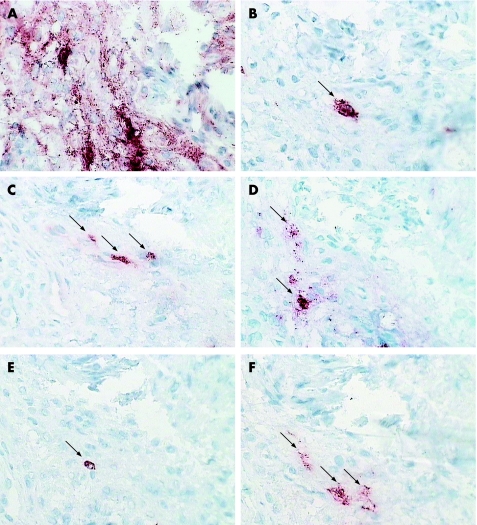
STAT4 co‐localised with BDCA‐2, a marker specific for PDCs, whose expression decreases as the PDC undergoes maturation. We also found that STAT4 co‐localised with some CD123 (IL3 receptor) expression (fig 2). CD123 expression was also seen on numerous endothelial vessels. We observed co‐localisation with DEC‐205, a C‐type lectin receptor, using sequential staining, although we did not find evidence of co‐localisation with confocal microscopy (figs 2 and 3). DEC‐205 expression increases markedly with DC maturation, although its function in PDCs is unknown.
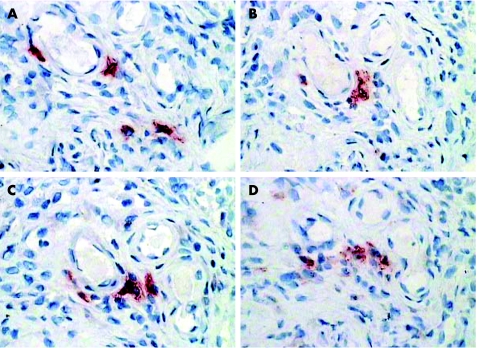
Figure 2 Sequential sections from the synovial membrane of a patient with RA stained for STAT4 (A), CD83 (B), CD123 (C), DEC‐205 (D), BDCA‐2 (E) and interferon‐α (F) using an immunoperoxidase method with AEC chromogen (red). Cells which co‐localise with STAT4 labelling are marked with arrows. Magnification ×400.
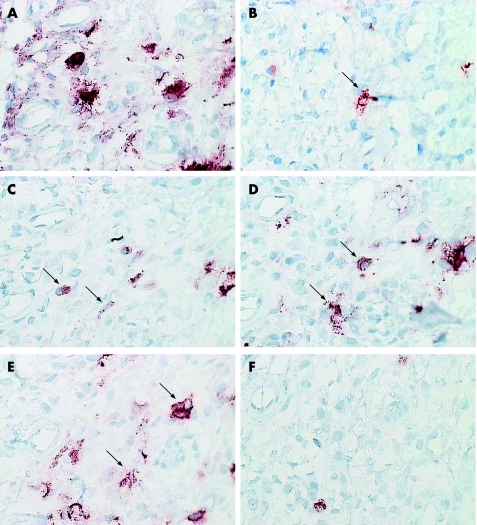
Figure 3 Sequential sections from the synovial membrane of a patient with RA (different patient from fig 2), stained for STAT4 (A), CD83 (B), DEC‐205 (C), CD1a (D), CMRF56 (E) and p55 (F) using an immunoperoxidase method with AEC chromogen (red). Cells which co‐localise with STAT4 labelling are marked with arrows. Magnification ×400.
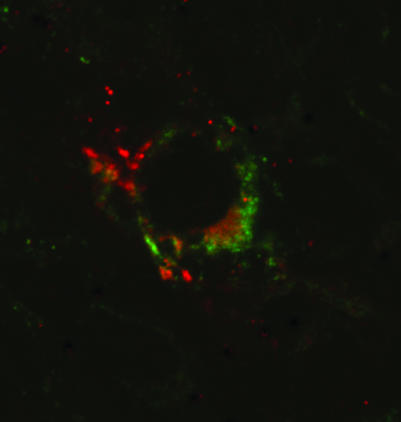
We found strong evidence of co‐localisation with CMRF56, an early activation marker of DCs (fig 3). We observed occasional co‐localisation of Jak3 and STAT4 bright cells with CD83 and p55 (mature DC markers) using a combination of sequential immunohistochemical labelling and confocal microscopy (figs 3 and 4).
Figure 4 Confocal microscopy of RA synovial membrane stained for STAT 4 (FITC, green) and CD83 (Cy3, red) by immunofluorescence to demonstrate co‐localisation. Magnification ×600, digitally enhanced to ×1800.
Many bright cells were observed within or surrounding lymphoid collections, but we were unable to demonstrate any co‐localisation of bright Jak/STAT cells with R4/23, an interdigitating reticulum cell marker.
Cytokine production by Jak3 and STAT4 bright cells
To confirm our findings that the Jak3/STAT4 bright cells are a population of mature or maturing DCs, we assessed cytokine production by these cells, using immunohistochemistry. Mature DCs produce large amounts of IL12 and IFNγ while mature PDCs produce IFNα and possibly IL12. We found that some but not all Jak3/STAT4 bright cells co‐localised with IL12 and IFNγ (fig 5). When co‐localisation did not occur, the cells were often in close vicinity to one another. IFNα also showed intermittent co‐localisation with Jak3 and STAT4 (fig 2).
Figure 5 Sequential sections from the synovial membrane of a patient with RA stained for STAT4 (A), Jak3 (C), IL12 (B) and interferon‐γ (D) using an immunoperoxidase method with AEC chromogen (red). Magnification ×200.
In summary, cytokine production in the Jak3/STAT4 bright cells was consistent with two populations of DCs—namely, plasmacytoid and myeloid or conventional DCs.
Discussion
Both immature and mature DC subsets occur within the rheumatoid synovium. Immature DCs (CD1a+) were found to be preferentially localised to the lining and sublining regions in biopsy specimens from 10/12 patients undergoing joint synovectomy.23 Another group using tissue taken at the time of joint replacement surgery only detected CD1a+ cells in 25% of patients.24 These variations may relate to differences in disease activity between the two biopsy groups. Mature DCs are found in association with perivascular and lymphocytic infiltrates.23,25 Where direct comparison of immature and differentiated DCs was undertaken within the one study a relative accumulation of immature DCs was observed, suggesting a relative defect in DC maturation in the synovium.23 More recently, two groups have reported on the presence of PDCs within the rheumatoid synovium and synovial fluid. Both found that synovial fluid derived PDCs were relatively immature, probably as a result of local inhibitory factors. In contrast, PDCs within the synovium showed evidence of a mature phenotype based on the increased preponderance of CD123+ to BDCA‐2+ cells (BDCA‐2 is downregulated upon maturation) and their association with MxA, a protein specifically induced by IFNα, and therefore suggesting a more mature DC type.26,27
In representative rheumatoid tissues, we have found in this study that the Jak3/STAT4 brightly staining cells co‐localised intermittently with moDCs (CD1a+) and PDCs (BDCA‐2+, CD123+) as well as early (CMRF56) and later DC maturation markers (CD83, p55, DEC‐205). Although there is some overlapping expression of these DC markers between different DC populations (see table 2), our results suggest that Jak3/STAT4 bright cells correspond to populations of MoDCs and PDCs at varying stages of maturation. It has been previously shown that CD40–CD154 ligand interaction results in upregulation of Jak3,17 with subsequent signalling through STAT6. STAT4 is the main signal transduction pathway for IL12, a cytokine produced by DCs to modulate immune responses. Therefore upregulation of these proteins in the same cell population, as we have shown in this study and previously,19 would be consistent with DCs undergoing activation.
We have previously shown that these cells did not co‐localise with any of the cell lineage markers generally observed in rheumatoid synovium but did intermittently co‐localise with CD1a, an immature DC marker. It is important to note that although CD1a is most strongly expressed in immature DC populations, it is still moderately present in intermediate mature DCs28 and must therefore be interpreted in context with other markers of maturation. Functional studies of these DCs would be required to establish precisely what we are recognising in the RA synovial tissue. We did not observe uniform co‐localisation with any particular DC marker, suggesting that intense Jak3/STAT4 expression reflects the degree of activation of DCs rather than any specific DC subpopulation. Unlike previous groups reporting on PDCs,26,27 we did not observe a preponderance of CD123+ to BDCA‐2+ cells in synovial tissue. Other staining patterns were similar to those previously described.23,29 In addition, we observed intermittent co‐localisation of Jak3 and STAT4 bright cells with staining for cytokines produced by both mature moDCs and PDCs (IFNγ, IL12 and IFNα). These observations support our hypothesis that these are DCs undergoing activation. Our findings are consistent with another group who found high levels of STAT4 expression in mature murine DCs. They also observed that autocrine activation of DCs by IL12 was essential for high‐level IFNγ production.4 It was not possible to test directly in tissue the validity of our proposal that intense expression of Jak3 and STAT6 could be explained by activation of Jak3 and subsequent STAT6 signalling through the CD40–CD154 ligand interaction,17 but our findings are consistent with this sequence of events.
Two other potential explanations for the changes we have observed merit consideration. In addition to its expression after CD40 ligation,17 Jak3 and also STAT6 have been found to have important roles in IL4 signalling in murine dendritic cells. Maturation of DCs induced by IL4 and IL13 occurred mainly through the type II IL4 receptor (IL4R) and was dependent upon STAT6 signalling, whereas enhancement of IL12 production by DCs occurred exclusively through the type I IL4R and was dependent upon Jak3.30 This provides another explanation for our observtion of the intense expression of Jak3 and STAT6 within the same cell.
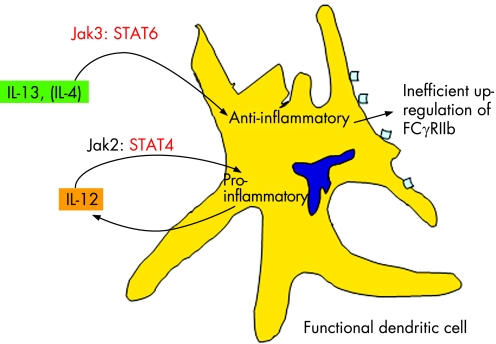
Finally, an alternative explanation for our findings may relate to signalling through the FCγ receptor (FCγR). FCγR are IgG‐specific receptors expressed by DCs. In humans they are divided into three classes (FCγRI, FCγRII and FCγRIII). FCγRII and FCγRIII interact preferentially with immune complexes. The FCγRII can be further divided into FCγRIIa and FCγRIIb receptors, with FCγRIIb being inhibitory.31 In murine models, deficiency of the FCγRIIb receptor has been shown to be associated with the production of RF.32 In human RA, FCγRII expression is increased and skewed towards the inhibitory subunit FCγRIIb. Despite this, RA DCs produce larger amounts of proinflammatory cytokines than DCs from normal subjects, possibly as a result of some disruption in the regulation of the pathway.31 IFNγ, IL4 and IL13 are known to influence FCγR expression, and IL13, in particular, is abundant in RA synovium. When compared with DCs from healthy controls, IL13 failed to increase FCγRII expression.31 On the basis of these findings, Radstake and coworkers have recently proposed that FCγRIIb expression is ineffectively upregulated in RA, resulting in a failure to inhibit the proinflammatory response generated by immune complexes.31 It is therefore possible that these Jak3/STAT4‐expressing DCs reflect an aberrant regulation pathway specific to RA, resulting in intense expression of Jak3/STAT4/STAT6, possibly related to impaired regulation of FCγR signalling. Jak3 and STAT6 expression might be increased in response to ineffective attempts to upregulate FCγRIIb expression by IL13 (or IL4), whereas STAT4 expression is consistent with continuing IL12 production in mature DCs (fig 6). Deficiencies in FCγRIIb have been linked to autoantibody production32 and provide an explanation for the association of these cells with the presence of RF.
Figure 6 In the later stages of the immune response, anti‐inflammatory cytokines such as interleukin (IL)4 and IL13 are released to reduce the inflammatory response. This leads to an increase in expression of FCγRIIb, an inhibitory FCγR subtype. In rheumatoid arthritis, dendritic cells may be inappropriately active in the late immune response as a result of inefficient signalling through IL13 and subsequent failure to increase FCγRIIb expression. If signalling by IL13 is inefficient, there will be a relative increase in Jak3 and STAT6 as the immune response attempts to downregulate its proinflammatory effects. The absence of successful inhibition will lead to continuing production of IL12 and its autocrine and paracrine proinflammatory effects. At the level of transcription pathways, this would be manifest by increased expression of STAT4.
In conclusion, we have identified a population of mature or maturing DCs specific to RA and particularly associated with the presence of serum RF, which intensely express Jak3, STAT4 and STAT6. The expression of these signal transduction molecules may reflect a pathogenic process unique to RA. Our findings raise the possibility of using therapeutic agents targeted to the Jak/STAT pathway as a means of modulating DC function in RA.
Acknowledgements
This research was aupported by the Daw Park Research Foundation, National Health and Medical Research Council of Australia and the Arthritis Foundation of Australia.
Abbreviations
CRP - C reactive protein
DCs - dendritic cells
mo DCs - myeloid DCs
DMARD - disease‐modifying antirheumatic drug
IFN - interferon
IL - interleukin
MHC - histocompatibility complex
PDCs - plasmacytoid dendritic cells
RA - rheumatoid arthritis
RF - rheumatoid factor
References
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature 1998392245–252. [DOI] [PubMed] [Google Scholar]
- 2.Adams S, O'Neill D W, Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol 200525177–188. [DOI] [PubMed] [Google Scholar]
- 3.Frucht D M, Fukao T, Bogdan C, Schindler H, O'Shea J J, Koyasu S. IFN‐γ production by antigen‐presenting cells: mechanisms emerge. Trends Immunol 200122556–560. [DOI] [PubMed] [Google Scholar]
- 4.Fukao T, Frucht D M, Yap G, Gadina M, O'Shea J J, Koyasu S. Inducible expression of STAT4 in dendritic cells and macrophages and its critical role in innate and adaptive immunity. J Immunol 20011664446–4455. [DOI] [PubMed] [Google Scholar]
- 5.Gad M, Claesson H M, Pedersen A E. Dendritic cells in peripheral tolerance and immunity. APMIS 2003111766–775. [DOI] [PubMed] [Google Scholar]
- 6.Rossi M, Young J W. Human dendritic cells: potent antigen‐presenting cells at the crossroads of innate and adaptive immunity. J Immunol 20051751373–1381. [DOI] [PubMed] [Google Scholar]
- 7.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F.et al BDCA‐2, a novel plasmacyotid dendritic cell‐specific type II C‐type lectin, mediates antigen capture and is a potent inhibitor of interferon‐α/β. J Exp Med 20011941823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colonna M, Trinchieri G, Liu Y J. Plasmacytoid dendritic cells in immunity. Nature Immunol 200451219–1226. [DOI] [PubMed] [Google Scholar]
- 9.McKenna K, Beignon A S, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol 20057917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Garra A, Trinchieri G. Are dendritic cells afraid of commitment? Nature Immunol 200451206–1208. [DOI] [PubMed] [Google Scholar]
- 11.Radstake T R D J, van Lieshout A W T, van Riel P L C M, van den Berg W B, Adema G J. Dendritic cells, Fcγ receptors, and toll‐like receptors: potential allies in the battle against rheumatoid arthritis. Ann Rheum Dis 2005641532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas R, Davis L S, Lipsky P E. Rheumatoid synovum is enriched in mature antigen‐presenting cells. J Immunol 19941522613–2623. [PubMed] [Google Scholar]
- 13.Kremer J M, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S.et al Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. N Engl J Med 20033491907–1915. [DOI] [PubMed] [Google Scholar]
- 14.Roelofs M F, Boelens W C, Joosten L A B, Abdoolahl‐Roodsaz S, Geurts J, Wunderink L U.et al Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 20061767021–7027. [DOI] [PubMed] [Google Scholar]
- 15.Morita Y, Yang J, Gupta R, Shimizu K, Shelden E A, Endres J.et al Dendritic cells genetically engineered to express IL‐4 inhibit murine collagen‐induced arthritis. J Clin Invest 20011071275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cetkovic‐Cvrlje M D A, Vassilev A, Liu X, Uckun F M. Targeting JAK3 with JANEX‐1 for prevention of autoimmune type 1 diabetes in NOD mice. Clin Immunol 2003106213–225. [DOI] [PubMed] [Google Scholar]
- 17.Säemann M D, Diakos C, Kelemen P, Kriehuber E, Zeyda M, Böhmig G A.et al Prevention of CD40‐triggered dendritic cell maturation and induction of T cell hyporeactivity by targeting of janus kinase 3. Am J Transplant 200331341–1349. [DOI] [PubMed] [Google Scholar]
- 18.Walker J G, Smith M D. The Jak‐STAT pathway in rheumatoid arthritis. J Rheumatol 2005321650–1653. [PubMed] [Google Scholar]
- 19.Walker J G, Ahern M J, Coleman M, Weedon H, Papangelis V, Beroukas D.et al Expression of Jak3, STAT1, STAT4 and STAT6 in inflammatory arthritis: unique Jak3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis 200665149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 21.Smith M, Chandran G, Youssef P, Darby T, Ahern M. Day case knee arthroscopy under regional anaesthesia performed by rheumatologists. Aust NZ J Med 199626108–109. [DOI] [PubMed] [Google Scholar]
- 22.Wikaningrum R, Highton J, Parker A, Coleman M, Hessian P A, Roberts‐Thomson P J.et al Pathogenic mechanisms in the rheumatoid nodule: comparison of proinflammatory cytokine production and cell adhesion molecule expression in rheumatoid nodules and synovial membranes from the same patient. Arthritis Rheum 1998411783–1797. [DOI] [PubMed] [Google Scholar]
- 23.Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol 20021685333–5341. [DOI] [PubMed] [Google Scholar]
- 24.Cauli A, Pitzalis C, Yanni G, Awad M, Panayi G S. CD1 expression in psoriatic and rheumatoid arthritis. Rheumatology (Oxford) 200039666–673. [DOI] [PubMed] [Google Scholar]
- 25.Pettit A R, MacDonald K P A, O'Sullivan B, Thomas R. Differentiated dendritic cells expressing nuclear RelB are predominantly located in rheumatoid synovial tissue perivascular mononuclear cell aggregates. Arthritis Rheum 200043791–800. [DOI] [PubMed] [Google Scholar]
- 26.Cavanagh L L, Boyce A, Smith L, Padnamabha J, Filgeuira L Pietschmann P.et al Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res Ther 20047R230–R240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lande R, Giacomini E, Serafini B, Rosicarelli B, Sebastiani G D, Minisola G.et al Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J Immunol 20041732815–2824. [DOI] [PubMed] [Google Scholar]
- 28.Bykovskaia S N, Shurin G V, Graner S, Bunker M L, Olson W, Thomas R.et al Differentiation of immunostimulatory stem‐cell and monocyte‐derived dendritic cells involves maturation of intracellular compartments responsible for antigen presentation and secretion. Stem Cells 200220380–393. [DOI] [PubMed] [Google Scholar]
- 29.Pettit A R, Ahern M J, Zehntner S, Smith M D, Thomas R. Comparison of differentiated dendritic cell infiltration of autoimmune and osteoarthritis synovial tissue. Arthritis Rheum 200144105–110. [DOI] [PubMed] [Google Scholar]
- 30.Lutz M B, Schnare M, Menges M, Rössner S, Röllinghoff M, Schuler G.et al Differential functions of IL‐4 receptor types I and II for dendritic cell maturation and IL‐12 production and their dependency on GM‐CSF. J Immunol 20021693574–3580. [DOI] [PubMed] [Google Scholar]
- 31.Radstake T R D J, Nabbe K C A M, Wenink M H, Roelofs M F, Oosterlaar A, van Lieshout A W T.et al Dendritic cells from RA patients lack the IL‐13 mediated increase of FcγRII expression, which has clear functional consequences. Ann Rheum Dis 2005641737–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moll T, Nitschke L, Carroll M, Ravetch J V, Izui S. A critical role for Fc γ RIIB in the induction of rheumatoid factors. J Immunol 20041734724–4728. [DOI] [PubMed] [Google Scholar]