Abstract
Objectives
To assess the impact of the intra‐articular distribution of 90yttrium‐citrate (90Y) on the clinical effect of radiosynoviorthesis (RSO) of the knee and on 90Y leakage from this joint.
Methods
Patients with arthritis of the knee received 185 MBq 90Y combined with a glucocorticoid, followed by clinical bed rest. Intra‐articular 90Y distribution, measured with a dual‐head gamma camera immediately or after 24 hours, was scored as mainly diffuse or mainly focal. Leakage to regional lymph nodes, the liver and spleen was assessed with a dual‐head gamma camera after 24 hours. Clinical effect was scored after 6 months by a composite change index (CCI), range 0–12; responders were defined as having a CCI ⩾6.
Results
Seventy‐eight knees of 69 patients, mostly suffering from undifferentiated arthritis (42%) or RA (28%), were treated. 90Y distribution was mainly diffuse in 54% and mainly focal in 46% with clinical response rates of 40% versus 56%, respectively, p = 0.3. CCI was not correlated with distribution. 90Y leakage was found only to the liver and the spleen (mean leakage 0.4% and 1.1%, respectively). Leakage was significantly less in case of diffuse intra‐articular 90Y distribution, whereas leakage to the liver was correlated with distribution (r = 0.68, p<0.001). 90Y leakage was not correlated with CCI.
Conclusions
Intra‐articular 90Y distribution does not influence the clinical effect of RSO of the knee. Although 90Y leakage from the joint is less if 90Y distributes diffusely in the joint cavity, leakage does not seem to hamper the clinical effect.
Radiation synovectomy or radiosynoviorthesis (RSO) is a therapeutic option for persistent arthritis of the knee, performed by intra‐articular administration of 90Yttrium (90Y).1,2 The mechanism of action is local radiation inducing necrosis of the synovial membrane, followed by fibrosis and sclerosis. After injection of 90Y, the needle is flushed with glucocorticoids (GC) to prevent a chemically induced flare‐up of arthritis and reflux of 90Y, and to help to bridge the lag phase before the effect of RSO, which is assumed to last 3–6 months.1,3 GC could also reduce leakage of 90Y into the blood by reducing within several hours synovitis and associated hypervascularity. It would seem plausible that a diffuse 90Y distribution would predict a better outcome of RSO than focal distribution,4 because of less surface contact between 90Y and the inflamed synovial tissue in the latter situation. However, whether the distribution of 90Y in the joint cavity after the injection is of importance for the clinical effect of RSO, has sparsely been investigated.
The aim of this study was to investigate the impact of the intra‐articular 90Y distribution on the clinical outcome of RSO and on its leakage from the joint.
Patients and methods
Patients
In a Dutch randomised clinical trial (RCT) the clinical effect of intra‐articular 90Y and GC versus that of GC was compared.5 Detailed data on the RCT have been described elsewhere.5 Main outcome measure was a composite change index (CCI) (range 0–12), assessed 6 months after therapy being the lag phase of 90Y.6 The CCI included a functional disability score, Visual Analogue Scale of pain, joint tenderness, swelling and effusion of the knee, and patient's and physician's global assessment of the effect of therapy. Successful therapy was defined as CCI ⩾6. Crossover therapy was applied afterwards if there was treatment failure. The subgroups of RSO patients in whom distribution (n = 78) and leakage (n = 33) of 90Y were assessed are the subject of this paper. RSO was applied using 185 MBq yttrium‐90 citrate (CIS bio international) and either 20 mg triamcinolone hexacetonide or 40 mg triamcinolone acetonide. After injection, the knee was bent several times,7 followed by splinting and 72 hours clinical bed rest.8
Methods
Assessments
a) distribution
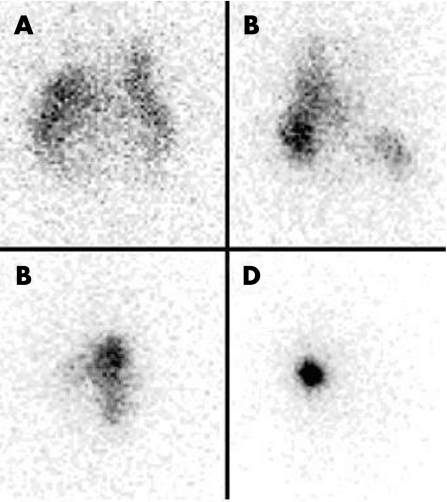
Distribution was assessed in four centres, either immediately after RSO (one centre) or after 24 hours. The European Association of Nuclear Medicine (EANM) guidelines for radiosynovectomy9 lack a guideline on the interval of time between injection and assessment of distribution. 90Y distribution was imaged with a Philips Vertex MCD dual‐head gamma camera equipped with VXHR collimators, registering ‘bremsstrahlung' during 10 min in a energy window (69 KeV ± 10%) and in a 256×256 matrix. Distribution was scored by the involved nuclear physician as follows: I) diffuse, II) predominantly diffuse, but also focal, III) predominantly focal, but also diffuse, or IV) focal (fig 1). There were no patients with extra‐articular distribution. For some analyses, the four classes were compacted into two groups: ‘mainly diffuse' (classes I&II) or ‘mainly focal' distribution (classes III&IV).
Figure 1 Examples of scoring intra‐articular distribution on the distribution scans into the four classes I–IV, A) diffuse (class I), B) predominantly diffuse, but also focal (class II), C) predominantly focal, but also diffuse (class III), D) focal (class IV) distribution. The contour of the knee joint can be estimated from 1A.
b) leakage
Since assessment of leakage is not required according to the EANM guidelines,9 leakage after 24 hours was measured in only three centres. Leakage was assessed with a Philips Vertex MCD dual‐head gamma camera equipped with VXHR collimators at inguinal lymph nodes (n = 15 RSOs), liver (n = 33 RSOs), and spleen (n = 18 RSOs). Counts in these regions were corrected for background radiation and expressed as percentage of counts corresponding with the whole injected dose.
Statistical analyses
The association between clinical effect and distribution pattern was explored by Spearman correlations, multiple linear regression analysis (dependent variable: CCI; independent variable: distribution classes I–IV by dummy variable coding) and logistic regression analysis (dependent variable: successful treatment “yes” or “no”).
The association between leakage and distribution was explored by Spearman correlations. In the two groups “mainly diffuse” or “mainly focal” distribution, leakage was tested for statistically significant difference. Because in only 18 knees both leakage and distribution were assessed, no regression analyses were performed.
Tests were two‐sided; p values <0.05 were considered to be statistically significant. Analyses were performed with Number Cruncher Statistical System 2000 and Statistical Package for Social Sciences, version 10.
Results
Baseline characteristics
In 69 patients 78 RSOs were performed; in nine patients both knees were treated. Patients suffered mainly from undifferentiated arthritis (42%) or RA (28%) (Table 1), and were 48±16 years old, range 19–76. Mean disease duration was 7±8 years, range 1–46, while mean duration of arthritis of the knee was 39±36 months, range 6–240. Most patients had pre‐existing radiological damage of the treated knee (Steinbrocker radiological class I 28%, class II 71% and class III 1%). In 33%, 90Y+GC was administered at crossover. At RSO, synovial fluid (SF) was successfully aspirated in 86%.
Table 1 The relation between intra‐articular 90Y distribution and the clinical effect of RSO, radiological class and leakage (n = 78 knees)*.
| Mainly diffuse distribution n = 42 | Mainly focal distribution n = 36 | p | |
|---|---|---|---|
| Composite change index (CCI, 0–12) | |||
| CCI, classified | n (%) | n (%) | 0.6 |
| 0–4 | 19 (45) | 12 (33) | |
| 4–8 | 15 (36) | 16 (45) | |
| 8–12 | 8 (19) | 8 (22) | |
| Median (95% LCL‐ 95% UCL) | 5 (3–7) | 7 (4–8) | 0.5 |
| Responders (CCI ⩾6), n (%) | 17 (40) | 20 (56) | 0.3 |
| Radiological class | n (%) | n (%) | 0.2 |
| I | 14 (35) | 7 (20) | |
| II | 25 (63) | 28 (80) | |
| Leakage (% of given dose)† | |||
| to the liver | |||
| Number of assessments | 18 | 15 | |
| mean (SD) | 0.2 (0.4) | 0.9 (0.8) | 0.0004 |
| leakage to liver, classified: | n | n | 0.004 |
| 0 | 15 | 3 | |
| 0–1 | 2 | 7 | |
| 1–2 | 1 | 4 | |
| 2–3 | 0 | 1 | |
| to the spleen | |||
| number of assessments | 5 | 13 | |
| mean (SD) | 0.6 (0.4) | 1.6 (1.3) | 0.09 |
| leakage to spleen, classified: | n | n | 0.2 |
| 0 | 0 | 1 | |
| 0–1 | 5 | 5 | |
| 1–2 | 0 | 4 | |
| 2–3 | 0 | 2 | |
| 3–4 | 0 | 0 | |
| 4–5 | 0 | 1 |
*78 knees: rheumatoid arthritis 22, undifferentiated arthritis 33, psoriatic arthritis 17, osteoarthritis 1, pigmented villonodular synovitis 1, synovial chondromatosis 1, ankylosing spondylitis 1, undifferentiated seronegative spondyloarthropathy 1, calcium pyrophosphate arthropathy 1.
†Counts, expressed as percentage of total counts corresponding with the whole injected dose.
Distribution
90Y distribution was “mainly diffuse” in 54% and “mainly focal” in 46%. Baseline variables were not different between these two groups. In RA, distribution was “mainly diffuse” in 64% versus 50% in non‐RA (ie, all other diagnoses than RA) (p = 0.3). The distribution pattern in patients in whom 24 hours after RSO distribution was assessed was not significantly different from the pattern in patients with an immediate assessment (p = 0.8). Distribution was more “mainly diffuse” if triamcinolone acetonide (n = 13) was co‐administered than if triamcinolone hexacetonide (n = 65) was used (92% versus 46%, p = 0.002), whereas there was a trend towards more “mainly diffuse” distribution if SF was aspirated than if not (57% versus 25%, p = 0.1). In regression analyses, only the type of co‐administered GC predicted distribution (r = −2.74, p = 0.03).
Clinical effect was not significantly different between the two groups (response rate 40% for “mainly diffuse” distribution versus 56% for “mainly focal”, p = 0.3, CCI 5±3 versus 6±4, respectively, p = 0.5), even if CCI was categorised (Table 1). CCI did not correlate with the four classes of distribution (r = 0.06, p = 0.6) and could neither be predicted by distribution. Neither clinical effect (p = 0.8) nor intra‐articular distribution (p = 1.0) were significantly different in the crossover group.
Leakage
No 90Y leakage to inguinal lymph nodes was found. Mean±SD leakage to the liver was 0.4±0.7%, range 0–2.5, and to the spleen 1.1±1.2%, range 0–5. Leakage to the liver (n = 33) was significantly less if “mainly diffuse” distribution (n = 18) than if “mainly focal” distribution (n = 15) was present: 0.2±0.4% versus 0.9±0.8%, p<0.0001. Similar results were found for leakage to the spleen (n = 18) (0.6±0.4% versus 1.6±1.3%, respectively, p = 0.04). Leakage was not different in the crossover group. Distribution (class I–IV) was correlated with leakage to the liver (r = 0.68, p<0.001) but not with leakage to the spleen (p = 0.1), while leakage to the liver and to the spleen were correlated (r = 0.86, p<0.0001). Clinical effect (CCI) was not correlated with leakage (counts) to the liver (p = 0.9) or to the spleen (p = 0.4).
Discussion
No association was found between intra‐articular distribution of 90Y and the clinical effect of RSO in nearly 80 knees, corresponding with data in the literature.10 However, one study showed a trend towards earlier relapse of arthritis of the knee after poor intra‐articular distribution,4 but in patients with hydroxyapatite arthropathy undergoing RSO with samarium‐153.
In the present study, despite bending of the knee several times after RSO for better intra‐articular dispersal of injected fluids,7 distribution was still “mainly diffuse” in only 54%. 90Y distribution appears to correspond to areas of increased synovial activity,10,11 but it seems unrealistic to assume that patients with “mainly focal” distribution had focal synovial inflammation in the knee. However, since all patients had persistent arthritis, intra‐articular septa or especially large synovial folds could have hampered the intra‐articular distribution. The distribution patterns in the different participating centres were not significantly different, indicating no influence of the injection technique on distribution. Unexpectedly, distribution was dependent on the type of GC used, perhaps due to the physical chemical properties of the different solvents or, less likely, of the compounds themselves. As distribution did not predict the clinical effect and as there are no measures to take if the distribution scan indicates that the injection technique failed, standard assessment of distribution according to EANM guidelines9 seems to serve no purpose.
Our leakage data are in accordance with the literature.11,12,13 Leakage did not seem to hamper the clinical effect of RSO, although assessed in a small group. Despite the fact that an increased frequency of chromosomal aberrations in circulating lymphocytes after RSO has been reported,890Y does not seem to increase the risk of cancer.14 So, in our opinion, the importance of leakage should not be overestimated. Whether RSO should be the treatment of first choice in the treatment of persistent arthritis of the knee, since in previous studies5,15 superiority of RSO over GC alone was debatable and long‐term results are limited, is discussed elsewhere.5
In conclusion, intra‐articular 90Y distribution does not influence the clinical effect of RSO of the knee. Although 90Y leakage from the joint is less if 90Y distributes diffusely in the joint cavity, leakage does not seem to hamper the clinical effect.
Acknowledgements
We would like to thank P Nauta, pharmacist of the department of nuclear medicine UMCU, for his comments on this manuscript. We are also grateful to the following rheumatologists for referring patients for inclusion in this study: AAM Blaauw, C van Booma‐Frankfort, GAW Bruyn, JC Ehrlich, AA van Everdingen, EN Griep, DM Hofman, PM Houtman, TL Jansen, KJ Korff, AA Kruize, JD Moolenburgh, HK Ronday, Y Schenk, WAA Swen, MJ van der Veen and CM Verhoef.
Abbreviations
CCI - composite change index
GC - glucocorticoids
RSO - radiosynoviorthesis
SF - synovial fluid
90Y - 90yttrium‐citrate
Footnotes
Funding: This work was supported by a grant from the Netherland's Organization for Scientific Research (NWO) 920‐03‐053.
Competing interests: None declared
References
- 1.Clunie G, Ell P J. A survey of radiation synovectomy in Europe, 1991–1993. Eur J Nucl Med 199522970–976. [DOI] [PubMed] [Google Scholar]
- 2.Schneider P, Farahati J, Reiners C. Radiosynovectomy in rheumatology, orthopedics, and hemophilia. J Nucl Med 200546(Suppl 1)48S–54S. [PubMed] [Google Scholar]
- 3.Jacob R, Smith T, Prakasha B, Joannides T. Yttrium90 synovectomy in the management of chronic knee arthritis: a single institution experience. Rheumatol Int 200323216–220. [DOI] [PubMed] [Google Scholar]
- 4.Clunie G, Lui D, Cullum I, Ell P J, Edwards J C. Clinical outcome after one year following samarium‐153 particulate hydroxyapatite radiation synovectomy. Scand J Rheumatol 199625360–366. [DOI] [PubMed] [Google Scholar]
- 5.Jahangier Z N, Jacobs J W G, Lafeber F P J G, Moolenburgh J D, Swen W A A, Bruyn G A W.et al Is radiation synovectomy for arthritis of the knee more effective than intra‐articular treatment with glucocorticoids? An 18 month randomized double‐blind placebo‐controlled cross‐over trial. Arthritis Rheum 2005523391–3402. [DOI] [PubMed] [Google Scholar]
- 6.Sledge C B, Atcher R W, Shortkroff S, Anderson R J, Bloomer W D, Hurson B J. Intra‐articular radiation synovectomy. Clin Orthop 198437–40. [PubMed]
- 7.Clunie G, Lui D, Cullum I, Edwards J C, Ell P J. Samarium‐153‐particulate hydroxyapatite radiation synovectomy: biodistribution data for chronic knee synovitis. J Nucl Med 19953651–57. [PubMed] [Google Scholar]
- 8.De la Chapelle A, Oka M, Rekonen A, Ruotsi A. Chromosome damage after intra‐articular injections of radioactive yttrium. Effect of immobilization on the biological dose. Ann Rheum Dis 197231508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clunie G, Fischer M. EANM procedure guidelines for radiosynovectomy. Eur J Nucl Med Mol Imaging 200330BP12–BP16. [DOI] [PubMed] [Google Scholar]
- 10.Kyle V, Hazleman B L, Wraight E P. Yttrium‐90 therapy and 99MTc pertechnetate knee uptake measurements in the management of rheumatoid arthritis. Ann Rheum Dis 198342132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gratz S, Gobel D, Behr T M, Herrmann A, Becker W. Correlation between radiation dose, synovial thickness, and efficacy of radiosynoviorthesis. J Rheumatol 1999261242–1249. [PubMed] [Google Scholar]
- 12.Rekonen A, Kuikka J, Oka M. Retention and extra‐articular spread of intra‐articularly injected 90Y silicate. Scand J Rheumatol 1976547–48. [PubMed] [Google Scholar]
- 13.Jaworski R, McLean R, Choong K, Smart R, Edmonds J. Re‐evaluating the need for hospitalization following synovectomy using Yttrium‐90 silicate. Br J Rheumatol 1993321012–1017. [DOI] [PubMed] [Google Scholar]
- 14.Vuorela J, Sokka T, Pukkala E, Hannonen P. Does yttrium radiosynovectomy increase the risk of cancer in patients with rheumatoid arthritis? Ann Rheum Dis 200362251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuft‐Dorenbosch L L, de Vet H C, van der Linden S. Yttrium radiosynoviorthesis in the treatment of knee arthritis in rheumatoid arthritis: a systematic review. Ann Rheum Dis 200059583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]