Abstract
Objective
The pro‐inflammatory calcium‐binding protein S100A12 has been recently ascribed to the novel group of damage associated molecular pattern (DAMP) molecules. Serum levels of S100A12 reflect neutrophil activation during synovial inflammation. The aim of this project was to analyse the effect of intra‐articular corticosteroids or systemic anti‐TNF treatment on synovial expression and serum levels of S100A12 in rheumatoid arthritis (RA).
Methods
Serum and synovial tissue was obtained from 19 RA patients prior to and 2 weeks after intra‐articular corticosteroid therapy. Serum was collected for 34 other patients, and in 14 of these patients synovial tissue was additionally obtained prior to and after 8 weeks of infliximab treatment. The expression of S100A12 was analysed by immunohistochemistry on frozen sections. Levels of S100A12 in serum were determined by ELISA.
Results
S100A12 serum levels were elevated in patients with active RA prior to therapy and decreased significantly in patients who responded to treatment in both patient groups, but not in non‐responders. The synovial expression of S100A12 was reduced 2 weeks after successful intra‐articular corticosteroid treatment. A similar decrease in local expression was found after 8 weeks of successful infliximab treatment.
Conclusions
Successful treatment of RA leads to downregulation of the DAMP protein S100A12. Expression and secretion of S100A12 is rapidly diminished after therapy with intra‐articular corticosteroids or infliximab. Taking these findings together, decreasing serum concentrations of S100A12 could reflect alleviated synovial neutrophil activation during successful anti‐inflammatory therapy in RA.
The innate immune system has a key role in host defence and in initiating inflammation. Therapies targeting cytokines mainly produced by phagocytes improve the clinical course of rheumatoid arthritis.1,2 Recently, a novel group of important pro‐inflammatory molecules has been introduced to the concept of innate immunity. In parallel to pathogen associated molecular patterns (PAMPs) as exogenous factors initiating inflammation, the term damage associated molecular pattern (DAMP) proteins for endogenous molecules that are released by activated or damaged cells under conditions of cell stress, has been established.3 An example of this group are phagocytic S100 proteins, which mediate inflammatory responses.4
S100A12 (calgranulin C) is a member of the S100 family of calcium‐binding proteins and has been described to define a novel pro‐inflammatory axis by binding to the multiligand receptor for advanced glycation end products (RAGE).5 S100A12 is expressed and secreted by activated granulocytes.6,7,8 Interaction of S100A12 with RAGE activates endothelial cells, macrophages and lymphocytes, and blocking of RAGE in experimental models of arthritis leads to suppression of the inflammatory response.9,10,11 Downstream signalling via the nuclear factor (NF)‐κB pathway results in the synthesis and secretion of pro‐inflammatory mediators.5
Rheumatoid arthritis (RA) is a chronic auto‐inflammatory disease characterised by synovial inflammation and progressive bone destruction that leads to joint deformity and physical disability.12 Tumour necrosis factor alpha (TNFα) has a central role in the pathogenesis of RA,13,14,15 as demonstrated by the clinical benefit of TNFα‐neutralising therapy.16,17,18 Sustained clinical benefit in patients with RA receiving a chimeric anti‐TNF antibody (infliximab) has been reported, but the exact molecular mechanism of this biological agent is still only partially understood.19 However, some of its beneficial effects could be related to the induction of apoptosis in monocytes and reduction of neutrophil activation.20,21,22,23
S100A12, as a marker of neutrophil activation, is released during inflammatory conditions at the site of inflammation.24 In a previous study, we showed synovial expression of phagocytic S100A12 in patients with active RA. Additionally, S100A12 concentrations in serum correlated strongly with synovial fluid levels and with disease activity in individual patients.25 In the present study, we focused on the expression of the damage associated molecule S100A12 in RA after anti‐TNF treatment and after intra‐articular corticosteroid therapy. We investigated the S100A12 expression in the synovium and in serum after treatment either directed against innate immune mechanisms or as a local immunosuppressive agent.
Methods
Patients
A total of 53 consecutive patients (40 female, 13 male; median age 51 years, range 21–83 years; table 1) were studied. All patients had active arthritis and fulfilled the American College of Rheumatology criteria of RA. Of the 53 patients, 33 were recruited at the Karolinska Hospital, Stockholm, Sweden and 20 at the Ghent University Hospital, Ghent, Belgium. The study protocol was approved by the Karolinska Hospitals Ethics Committee, Stockholm and by the Ethics Committee of the Ghent University Hospital respectively. Informed consent was obtained from each patient prior to the study.
Table 1 Characteristics of the patients treated with intra‐articular corticosteroids (IAC) or infliximab.
| Treatment groups | ||
|---|---|---|
| IAC | Infliximab | |
| Age, median (range), years | 51.2 (21–83) | 50.5 (25.0–74.0) |
| Female/male | 15/4 | 25/9 |
| Disease duration, median (range), years | 7.5 (0.3–23) | 10.8 (0.6–30.0) |
| S100A12 serum levels before therapy, ng/ml (±SEM) | 585 (±170) | 430 (±65) |
| S100A12 serum levels after therapy, ng/ml (±SEM) | 490 (±160) | 290 (±55) |
| CRP before therapy, mg/dl (±SEM) | – | 3.2 (±0.9) |
| CRP after therapy, mg/dl (±SEM) | – | 1.0 (±0.3) |
| ESR before therapy, mm/h (±SEM) | – | 23 (±4) |
| ESR after therapy, mm/h (±SEM) | – | 11 (±2) |
| Number of patients with concomitant therapy: | ||
| MTX (>7.5 mg/week) | 7/19 | 26/34 |
| Systemic corticosteroids | 4/19 | 14/34 |
–, value not determined.
A total of 19 patients received intra‐articular corticosteroids (IAC) as therapy. Per patient, only one knee joint was injected with triamcinolone hexacetonide 40 mg at one time. A total of 34 patients were treated with infliximab infusions, 3 mg/kg, at weeks 0, 2 and 6. For additional therapy information, see table 1. In the treatment group with IAC, biopsy specimens of the injected knee joints were obtained at baseline and 9–12 days after injection from all 19 patients. In the infliximab group, synovial biopsy samples were obtained from 14 of the 34 patients during arthroscopy before and after 8.8 weeks (range 2.7–17 weeks) of treatment. From all patients of this group serum samples were obtained at baseline and after a median of 7.2 weeks, i.e. after three infusions of infliximab.
Clinical and laboratory assessments
All patients were assessed for overall disease activity at baseline and during sample acquisition. Responders in the infliximab group were defined having at least 20% improvement (ACR 20) of clinical and laboratory parameters. In patients treated with IAC, response to therapy was defined as absence of joint swelling in clinical examination. In addition, significant reduced swelling on arthroscopic finding at days 9–12 in comparison to the first examination was noted in all these cases.
Biopsy specimens
Biopsy specimens were obtained by arthroscopy guided techniques in 33 patients. The biopsy specimens were taken from an area adjacent to the cartilage–pannus junction, placed in pre‐chilled isopentane on dry ice, and maintained at –70°C until sectioned. Several biopsies were taken from each biopsy‐site. From a representative single biopsy we stained the whole section and evaluated the infiltrate semiquantitatively in all fields of the section.
Tissue preparation and immunohistochemical staining
Cryostat sections (6–8 μm, cryostat setting 7 μm) were mounted on superfrost slides. Sections to be stained were initially fixed for 20 min with 2% (v/v) formaldehyde (Sigma Chemicals, St Louis, Missouri, USA) dissolved in phosphate buffered saline pH 7.4 at 4°C and were then left to dry before storage at −70°C.
Frozen sections of the biopsy specimen were stained to analyse the expression of S100A12. We used a polyclonal affinity purified rabbit antibody against human S100A12 prepared as reported previously.6 Monoclonal mouse antihuman granulocyte associated antigen CD15 antibody (Dako, Hamburg, Germany), a sensitive neutrophil marker, was used to detect neutrophils in infiltrates. Mouse antihuman CD68 (Dako, Hamburg, Germany) and mouse antihuman CD16326 were used to detect monocytes and macrophages. Staining on serial sections was performed to detect co‐expression of S100A12 and CD15 in infiltrates. For controls, monoclonal mouse IgM (Dianova, Hamburg, Germany) and polyclonal rabbit IgG (Amersham Biosciences, Freiburg, Germany) of irrelevant specificity were employed. Secondary antibodies and substrates for colour reaction were used as described previously.7,25
Sections were coded and analysed semiquantitatively on a four‐point scale (0–3) by two independent observers who were blinded to diagnosis and clinical data. The analysis included all areas of the sections at a magnification of 200‐fold and a global score was given for each parameter, using a semiquantitative four‐point scale (0 = lowest level of expression, no positive cells; 3 = highest level of expression, all cells positive in all areas).27 The summation index is a composite index that consists of the sum of the three layer indices.
Laboratory examinations
Concentration of S100A12 in the serum of patients was determined by a specific double‐sandwich enzyme‐linked immunosorbent assay (ELISA) system established in our laboratory as previously described.7 Polyclonal affinity purified rabbit antisera against human S100A12 were prepared as reported previously.6 Monospecificity of rabbit antihuman S100A12 antibody was analysed by immunoreactivity against purified human and recombinant S100A12, and Western blot analysis of lysates of granulocytes. The blood samples were centrifuged within 2 h and the serum was stored at −80°C until analysed. Serum concentrations of S100A12 are given as mean ± standard error of the mean (SEM) if not mentioned otherwise. C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were obtained from patients treated with infliximab for therapy monitoring when biopsies and serum samples were obtained. Blood samples for CRP and ESR were not taken routinely from patients who underwent local treatment.
Statistical analyses
Correlation was calculated using Spearman's correlation. Differences between groups were analysed using Mann–Whitney U test. Statistical analyses were performed using SPSS for Windows V.11.0. Data are presented as mean ± SEM except otherwise stated. p<0.05 was considered statistically significant.
Results
S100A12 serum concentrations
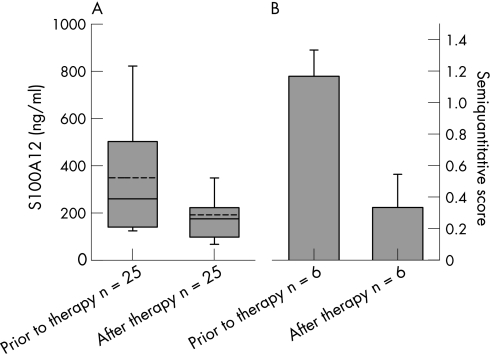
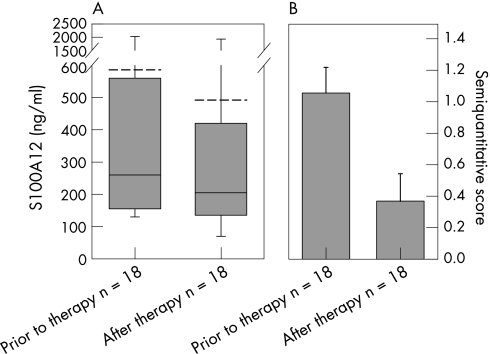
Serum samples of patients with active RA revealed a mean (±SEM) S100A12 concentration of 430±65 ng/ml in the infliximab group and of 585±170 ng/ml in the cortison group. After therapy, patients treated with infliximab had a mean concentration of 290±55 ng/ml (p<0.001). In the subgroup of patients who responded to infliximab therapy, mean serum levels fell from 350±50 ng/ml to 190±30 ng/ml after therapy (p = 0.001; fig 1A). Non‐responders had higher levels prior to therapy and did not show a significant decrease after therapy (650±180 ng/ml before and 620±150 ng/ml after therapy; p = 0.207; fig 1C). In the group of patients treated with intra‐articular corticosteroids, 18 of 19 (95%) patients showed clinical and arthroscopic response to therapy. Parallel to this response, we found a significant decrease of S100A12 serum levels after therapy from 585±170 ng/ml to 490±160 ng/ml (p = 0.031; fig 2A). Normal values of S100A12 serum levels are <120 ng/ml (lower than mean plus two standard deviations of healthy controls) as we have shown in previous studies.28 Concomitant therapy in both patient groups did not change during the follow‐up, thus S100A12 levels could not be affected by intraindividual varying medication.
Figure 1 (A) S100A12 serum levels (p = 0.001) and (B) S100A12 synovial sublining layer expression (p = 0.025) in patients who responded to infliximab treatment, before and after therapy. In contrast, S100A12 serum levels and S100A12 synovial sublining layer expression revealed no significant differences (p = 0.02 for both) in patients who did not respond to infliximab treatment (not shown). S100A12 serum levels are expressed as boxplots with median, 10th, 25th, 75th, 90th percentile and mean line (dashed line) and S100A12 synovial sublining layer expression as semiquantitative microscopic score. Mean ± SEM.
Figure 2 (A) S100A12 serum levels (p = 0.031) and (B) S100A12 synovial sublining layer expression (p = 0.002) in patients who responded to IAC treatment, before and after therapy. S100A12 serum levels are expressed as boxplots with median, 10th, 25th, 75th, 90th percentile and mean line (dashed line) and S100A12 synovial sublining layer expression as semiquantitative microscopic score. Mean ± SEM.
Synovial S100A12 expression and response to therapy
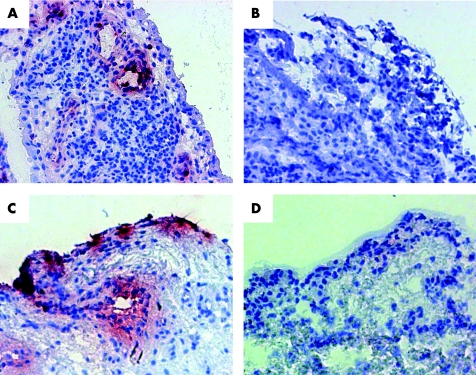
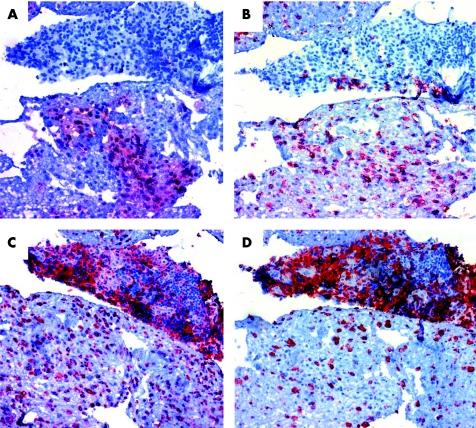
Immunohistochemical studies on the biopsies from inflamed synovia showed a specific pattern of S100A12 expression in inflamed areas. The S100A12 expression was most impressive in the sublining layer and in CD15 positive stained infiltrating cells (fig 3A, C). In addition, S100A12 was found in an extracellular distribution surrounding S100A12 positive cells, reflecting secretion of S100A12 and possibly binding to other RAGE bearing cells in infiltrates. As we could confirm in serial sections S100A12 positive granulocytes were CD15 positive, too. Coexpression of S100A12 with macrophage markers CD68 or CD163 could not be found (fig 4).
Figure 3 (A) Expression of S100A12 in synovial tissue before infliximab with response to therapy. (B) Synovial biopsy of the same patient after successful treatment showing markedly reduced S100A12 expression. (C) Expression of S100A12 in synovial tissue before IAC. (D) Synovial biopsy of the same patient after successful treatment showing markedly reduced S100A12 expression (peroxidase staining, red). The analysis included all areas of the sections at a magnification of 200‐fold.
Figure 4 Coexpression of S100A12 (A) and CD15 (B) as neutrophil marker in serial sections from a representative patient. In contrast, the expression‐pattern of the macrophage markers CD68 (C) or CD163 (D) differed substantially. The analysis included all areas of the sections at a magnification of 100‐fold.
The synovial expression of S100A12 was already significantly reduced 2 weeks after successful intra‐articular corticosteroid treatment as seen in the lining (p = 0.014) and the sublining layer (p = 0.002; fig 2B and table 2). A similar decrease in local expression was found after 8 weeks of infliximab treatment in responders. In the case of the sublining layer expression pattern, we could observe a significant decrease in S100A12‐positive cells (p = 0.025), whereas in the lining (p = 0.157) and perivascular layer (p = 0.234) the decrease was not significant (fig 1B and table 2). In patients who did not respond to infliximab therapy there was no significant decrease in any of the three layers (fig 1D and table 2).
Table 2 Mean S100A12 serum concentrations and expression of S100A12 in synovial tissue layers before and after treatment with intra‐articular Corticosteroids (IAC) or infliximab.
| Serum concentrations (ng/ml) | Lining layer | Sublining layer | Perivascular layer | Overall index | |
|---|---|---|---|---|---|
| IAC responder prior to IAC | 585 (±170) | 0.42 (±0.12) | 1.05 (±0.16) | 0.53 (±0.18) | 2.00 (±0.39) |
| After IAC | *490 (±160) | *0.11 (±0.07) | **0.37 (±0.18) | 0.32 (±0.13) | **0.79 (±0.35) |
| Infliximab responder prior to infliximab | 350 (±50) | 0.33 (±0.21) | 1.17 (±0.17) | 0.33 (±0.21) | 2.00 (±0.26) |
| After infliximab | **190 (±30) | 0 (±0) | *0.33 (±0.21) | 0 (±0) | *0.33 (±0.21) |
| Infliximab non‐responder prior to infliximab | 650 (±180) | 0.25 (±0.16) | 1.25 (±0.25) | 0.88 (±0.23) | 2.38 (±0.53) |
| After infliximab | 620 (±150) | 0 (±0) | 0.75 (±0.25) | 0.38 (±0.26) | 1.13 (±0.44) |
*p<0.05; **p<0.005.
Correlation between S100A12 serum concentrations and synovial expression
Serum levels of S100A12 and synovial expression as measured in a semiquantitative microscopic score correlated significantly for patients treated with infliximab and intra‐articular corticosteroids concerning the sublining and the perivascular layer (table 3). In the latter patient group there was also a correlation between S100A12 serum levels and lining layer expression.
Table 3 Correlations between S100A12 synovial expression and S100A12 serum concentrations.
| S100A12 synovial expression | S100A12 serum levels | |
|---|---|---|
| IAC treatment | Infliximab treatment | |
| Lining layer | *r = 0.52 (p = <0.001) | r = 0.04 (p = 0.85) |
| Sublining layer | *r = 0.54 (p = <0.001) | *r = 0.55 (p = 0.002) |
| Perivascular layer | *r = 0.36 (p = 0.014) | *r = 0.53 (p = 0.004) |
| Overall index | *r = 0.49 (p = 0.001) | *r = 0.55 (p = 0.002) |
*Significant correlation.
Discussion
A new concept of pattern recognition involves the multiligand RAGE in sensing endogenous DAMPs.3 Most DAMPs, i.e. HMGB1, heat‐shock proteins, uric acid, and S100 proteins, exhibit a double life; as intracellular molecules they have a role in cell homeostasis, e.g. as calcium‐binding proteins, chaperons, or chromatin‐stabilising molecules,4,29,30 but after release into the extracellular compartment as a result of cell damage, infections or inflammation they become danger signals that activate immune cells and vascular endothelium.3,31
In the present study, we investigated the DAMP molecule S100A12 and studied its differential expression levels in RA after anti‐TNF treatment and after intra‐articular corticosteroid therapy.
S100A12 and its interaction with the multiligand receptor RAGE represent a pro‐inflammatory axis within the innate immune system. The exact mechanisms involved in S100A12 secretion by neutrophils are poorly known, but there is evidence that S100A12 is secreted by activated neutrophils infiltrating the synovium.32,33 Upon secretion, S100A12 exhibits pro‐inflammatory functions after binding to RAGE on myelomonocytic cells and endothelium, thus stimulating inflammatory responses in synovial tissue via activation of NF‐κB and MAP kinase signal pathways.5,34 Data from murine models of inflammation demonstrated that S100A12 blocking antibodies could attenuate synovial inflammation.5,9,34 In these models, blocking the interaction of S100A12 with RAGE suppressed clinical and histologic evidence of arthritis, in parallel with diminished levels of TNF‐α, interleukin‐6 (IL‐6), and matrix metalloproteinases.5,9
We could confirm the previous observation that S100A12 is expressed in infiltrating CD15 positive neutrophils that assemble during inflammatory arthritis in the sublining layer of the synovial tissue. Expression of S100A12 in activated monocytes or macrophages has been reported under other inflammatory conditions, but we could not find coexpression of S100A12 with macrophage markers CD68 or CD163 in our synovia biopsies. In a previous study we have already shown reduced expression of S100A12 after methotrexate treatment in psoriatic arthritis.25 Now, we also add evidence for its differential expression in RA in response to infliximab and intra‐articular corticosteroids (IAC). Significant correlation between sublining layer S100A12 expression and S100A12 serum levels endorse the hypothesis that the origin of serum S100A12 is the protein secreted by these infiltrating granulocytes. Local expression of S100A12 leads to elevated synovial fluid levels in RA patients25 and subsequently to increased serum levels. Local treatment with immunosuppressive IAC induces a positive response to treatment in most RA patients; in our study, this was 95% of patients after 9–12 days. The normal response rate has been reported to be around 74% ACR 20 after 4 weeks, so our response rate was comparably rather high.35 Successful treatment leads to diminished expression of synovial S100A12 in the sublining layer and significantly lower S100A12 serum concentrations in parallel as quickly as 2 weeks after IAC therapy.
Monocytes and neutrophils as cells of the phagocytic lineage are extensively involved in synovial inflammation. Infiltration of phagocytes with production of various pro‐inflammatory cytokines results in synovitis.27,36,37,38 The importance of the innate immune system in chronic arthritis is emphasised by the fact that the most efficient available therapies, such as anti‐tumour necrosis factor (TNF) agents, are targeting phagocytes.23,39 Previous studies demonstrated the influence of TNF‐α on neutrophil infiltration in arthritis.40,41 We have demonstrated that TNF‐α also induces secretion of S100A12 in human neutrophils.7 Here, we demonstrate that successful direct blocking of TNF‐α in vivo reduces S100A12 expression and secretion in patients with RA and thus interrupts the positive feedback‐loop between TNF‐α and S100A12. Decreases of S100A12 serum levels indicated response to therapy in individual patients and correlated with the expression of S100A12 at local sites of inflammation. Measurement of serum S100A12 could therefore be a useful biological marker for anti‐cytokine therapy, i.e. infliximab. Patients were more likely to respond to infliximab when they had lower S100A12 levels, indicating that patients with higher S100A12 levels might require more aggressive treatment, e.g. more infliximab doses over time.
Interestingly, the expression level of S100A12 in the synovial tissue after a mean of 8 weeks treatment with infliximab is similar to the expression level after only 2 weeks of IAC therapy, but the serum concentrations are notably lower. IAC therapy is restricted to the site of inflammation and has almost no systemic effects. By contrast, the more significant decrease of serum S100A12 after infliximab could indicate that this therapy is capable of inhibiting systemic neutrophil activation in addition to the affected joints targeted by IAC.
While the exact pathogenetic role of S100A12 in RA still needs further evaluation, its overexpression in a variety of autoimmune and autoinflammatory diseases and a number of proven pro‐inflammatory effects of S100A12 in target cells underscore the relevance of this S100‐protein in human inflammatory disorders. It has been suggested that an initial unknown stimulus could be the starting signal for inflammatory arthritis and subsequently DAMPs as endogenous molecules lead to an amplification and chronification of inflammation.3,9
In conclusion, our data confirm that S100A12 is a factor present at local sites of inflammation as well as systemically expressed in RA and that this molecule is an excellent marker for monitoring anti‐inflammatory therapies directed either against local synovial inflammatory processes or systemic cytokine effects. For all RA standard therapies (MTX, infliximab, IAC) it is now proven that they have significant effects on S100A12 expression, indicating that S100A12 itself could be a direct target for successful anti‐inflammatory therapy in arthritis.
Acknowledgements
We thank Marianne Engström, Melanie Saers and Karin Bakey for excellent technical assistance.
Footnotes
This work was supported by grants from the Interdisciplinary Centre of Clinical Research (IZKF), University of Münster, Foe2/026/04, the Gustav V 80 years foundation, the Swedish Association Against Rheumatism and the “Åke Wiberg” Foundation.
Competing interests: None.
References
- 1.Bachmann M F, Kopf M. On the role of the innate immunity in autoimmune disease. J Exp Med 2001193F47–F50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldmann M, Maini R N. Anti‐TNF α therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 200119163–196. [DOI] [PubMed] [Google Scholar]
- 3.Lotze M T, Tracey K J. High‐mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 20055331–342. [DOI] [PubMed] [Google Scholar]
- 4.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte‐specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 200324155–158. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann M A, Drury S, Fu C, Qu W, Taguchi A, Lu Y.et al RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 199997889–901. [DOI] [PubMed] [Google Scholar]
- 6.Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C.et al S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem 199927425291–25296. [DOI] [PubMed] [Google Scholar]
- 7.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W.et al Neutrophil derived human S100A12 (EN‐RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 200352847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye F, Foell D, Hirono K I, Vogl T, Rui C, Yu X.et al Neutrophil‐derived S100A12 is profoundly upregulated in the early stage of acute Kawasaki disease. Am J Cardiol 200494840–844. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A M, Yan S D, Yan S F, Stern D M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001108949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim W, Hudson B I, Moser B, Guo J, Rong L L, Lu Y.et al Receptor for advanced glycation end products and its ligands: a journey from the complications of diabetes to its pathogenesis. Ann NY Acad Sci 20051043553–561. [DOI] [PubMed] [Google Scholar]
- 11.Bierhaus A, Humpert P M, Morcos M, Wendt T, Chavakis T, Arnold B.et al Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 200583876–886. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel S E. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 200127269–281. [DOI] [PubMed] [Google Scholar]
- 13.Brennan F M, Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol 19924754–759. [DOI] [PubMed] [Google Scholar]
- 14.Beckham J C, Caldwell D S, Peterson B L, Pippen A M, Currie M S, Keefe F J.et al Disease severity in rheumatoid arthritis: relationships of plasma tumour necrosis factor‐α, soluble interleukin 2‐receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D‐dimer to traditional severity and functional measures. J Clin Immunol 199212353–361. [DOI] [PubMed] [Google Scholar]
- 15.Charles P, Elliott M J, Davis D, Potter A, Kalden J R, Antoni C.et al Regulation of cytokines, cytokine inhibitors, and acute‐phase proteins following anti‐TNF‐α therapy in rheumatoid arthritis. J Immunol 19991631521–1528. [PubMed] [Google Scholar]
- 16.Elliott M J, Maini R N, Feldmann M, Kalden J R, Antoni C, Smolen J S.et al Randomised double‐blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 19943441105–1110. [DOI] [PubMed] [Google Scholar]
- 17.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, Macfarlane J D.et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumour necrosis factor α monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998411552–1563. [DOI] [PubMed] [Google Scholar]
- 18.Maini R, St Clair E W, Breedveld F, Furst D, Kalden J, Weisman M.et al Infliximab (chimeric anti‐tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 19993541932–1939. [DOI] [PubMed] [Google Scholar]
- 19.Lipsky P E, van der Heijde D M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumour Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 20.Pay S, Musabak U, Erdem H, Simsek I, Pekel A, Sengul A.et al Chimerical anti‐TNF‐α, infliximab, inhibits neutrophil chemotaxis and production of reactive oxygen species by blocking the priming effect of mononuclear cells on neutrophils. Immunopharmacol Immunotoxicol 200527187–198. [DOI] [PubMed] [Google Scholar]
- 21.Lugering A, Schmidt M, Lugering N, Pauels H G, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn's disease by using a caspase‐dependent pathway. Gastroenterology 20011211145–1157. [DOI] [PubMed] [Google Scholar]
- 22.Tak P P, Taylor P C, Breedveld F C, Smeets T J, Daha M R, Kluin P M.et al Decrease in cellularity and expression of adhesion molecules by anti‐tumour necrosis factor α monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum 1996391077–1081. [DOI] [PubMed] [Google Scholar]
- 23.Catrina A I, Trollmo C, af Klint E, Engstrom M, Lampa J, Hermansson Y.et al Evidence that anti‐tumour necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum 20055261–72. [DOI] [PubMed] [Google Scholar]
- 24.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum 2004503762–3771. [DOI] [PubMed] [Google Scholar]
- 25.Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C.et al Expression of the pro‐inflammatory protein S100A12 (EN‐RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003421383–1389. [DOI] [PubMed] [Google Scholar]
- 26.Zwadlo G, Voegeli R, Osthoff K S, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down‐regulatory phase of the inflammatory process. Exp Cell Biol 198755295–304. [DOI] [PubMed] [Google Scholar]
- 27.Kruithof E, Baeten D, De Rycke L, Vandooren B, Foell D, Roth J.et al Synovial histopathology of psoriatic arthritis, both oligo‐ and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res Ther 20057R569–R580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foell D, Frosch M, Sorg C, Roth J. Phagocyte‐specific calcium‐binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 200434437–51. [DOI] [PubMed] [Google Scholar]
- 29.van Eden W, van der Zee R, Prakken B. Heat‐shock proteins induce T‐cell regulation of chronic inflammation. Nat Rev Immunol 20055318–330. [DOI] [PubMed] [Google Scholar]
- 30.Dumitriu I E, Baruah P, Manfredi A A, Bianchi M E, Rovere‐Querini P. HMGB1: guiding immunity from within. Trends Immunol 200526381–387. [DOI] [PubMed] [Google Scholar]
- 31.Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T.et al Myeloid‐related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 20051052955–2962. [DOI] [PubMed] [Google Scholar]
- 32.Boussac M, Garin J. Calcium‐dependent secretion in human neutrophils: a proteomic approach. Electrophoresis 200021665–672. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Tao T, Raftery M J, Youssef P, Di Girolamo N, Geczy C L. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol 200169986–994. [PubMed] [Google Scholar]
- 34.Hofmann M A, Drury S, Hudson B I, Gleason M R, Qu W, Lu Y.et al RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun 20023123–135. [DOI] [PubMed] [Google Scholar]
- 35.Furtado R N, Oliveira L M, Natour J. Polyarticular corticosteroid injection versus systemic administration in treatment of rheumatoid arthritis patients: a randomized controlled study. J Rheumatol 2005321691–1698. [PubMed] [Google Scholar]
- 36.De Rycke L, Baeten D, Foell D, Kruithof E, Veys E M, Roth J.et al Differential expression and response to anti‐TNFα treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 200520617–27. [DOI] [PubMed] [Google Scholar]
- 37.Ulfgren A K, Grondal L, Lindblad S, Khademi M, Johnell O, Klareskog L.et al Interindividual and intra‐articular variation of proinflammatory cytokines in patients with rheumatoid arthritis: potential implications for treatment. Ann Rheum Dis 200059439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergunst C E, van de Sande M G, Lebre M C, Tak P P. The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol 200534415–425. [DOI] [PubMed] [Google Scholar]
- 39.Capsoni F, Sarzi‐Puttini P, Atzeni F, Minonzio F, Bonara P, Doria A.et al Effect of adalimumab on neutrophil function in patients with rheumatoid arthritis. Arthritis Res Ther 20057R250–R255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannetti C A, Leung B P, Culshaw S, McInnes I B, Cunha F Q, Liew F Y. IL‐18 enhances collagen‐induced arthritis by recruiting neutrophils via TNF‐α and leukotriene B4. J Immunol 20031711009–1015. [DOI] [PubMed] [Google Scholar]
- 41.Den Broeder A A, Wanten G J, Oyen W J, Naber T, van Riel P L, Barrera P. Neutrophil migration and production of reactive oxygen species during treatment with a fully human anti‐tumour necrosis factor‐α monoclonal antibody in patients with rheumatoid arthritis. J Rheumatol 200330232–237. [PubMed] [Google Scholar]