Abstract
Aim
To obtain iris biopsy samples of sufficient quality and quantity for histopathological analysis using a novel punch biopsy technique.
Methods
Two patients underwent iris tumour biopsy at an ocular oncology service. A trabeculectomy punch (Kelly Descemet's membrane punch) with a 1.0 mm diameter head and a 0.75 mm deep bite was inserted through a clear cornea perforated by a SatinSlit 3.2 mm angled slit knife into a viscoelastic‐filled anterior chamber. The Kelly punch was placed over the lesion and pressed down before the punch was made. After obtaining the sample, the Kelly punch was removed from the eye and then opened over a dry cellulose sponge. Tissue samples were placed in 4% formalin and processed routinely for standard staining with H&E, periodic acid Schiff and immunostains.
Results
In both patients, by using the punch biopsy technique with the Kelly punch, we were able to obtain a 0.8×0.6 mm piece of tissue, large enough for any histological analysis. H&E staining showed spindle cell melanoma. Tissue sections, stained positive with MART‐1 (melanoma antigen recognised by T cells) and negative with cytokeratin, established the diagnosis of melanoma of the iris in each of these patients.
Conclusions
Iris biopsy with the punch technique yields a tissue biopsy specimen, as opposed to cytology samples obtained by fine needle aspiration biopsy. This technique is quick, simple to perform and requires non‐expensive and easily available equipment. The tissue obtained is of sufficient quality and quantity to enable routine and special stainings.
Tumours in the anterior segment of the eye are much less common than in the posterior segment. The accessibility of these tumours enables us to take a biopsy specimen in cases where the diagnosis is uncertain. Fine needle aspiration biopsy (FNAB) of anterior segment tumours, whether solid or of floating cells in the aqueous fluid, was described by Czerniak and others.1,2,3,4 The limitations of the procedure include discrepancies in interpretation of the cytologic study and inadequate specimen.4 FNAB was therefore called fine‐needle aspiration cytopathology rather than biopsy by Wakely et al.5 In an attempt to increase the diagnostic accuracy of the biopsy, Bechrakis et al6 suggested the use of a vitrectomy probe to obtain a larger amount of tissue. This method has its own technical difficulties. The tissue samples are drawn into a large volume of fluid and have to be filtered out, and the cornea has to be sutured. The latter problem was solved by the use of a 25‐gauge aspiration cutter through a self‐sealing corneal incision.7 In both methods, expensive vitrectomy equipment was required.
In this paper, we describe a new technique for obtaining a large dry biopsy sample from iris lesions. Using a trabeculectomy Kelly Descemet's membrane punch, pieces of the tumour were removed and processed in paraffin wax sections for standard H&E and periodic acid Schiff staining, and for immunostainings.
Methods
Patients
Two patients who were referred to the ocular oncology clinic at the Hadassah University Hospital, Jerusalem, Israel, were evaluated for atypical tumours of the iris, in which the diagnosis was uncertain after clinical examination and high‐frequency ultrasonography. Informed consent was obtained from the patients. Institutional review board approval was not required for this study.
Surgical technique
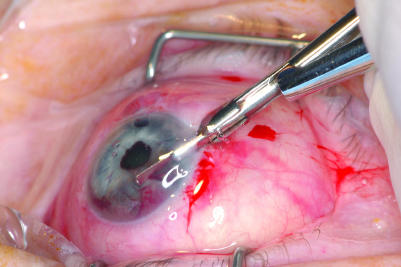
The punch biopsies were performed as an ambulatory procedure under local anaesthesia. A clear corneal incision with a SatinSlit 3.2 mm angled slit knife (Alcon, Fort Worth, Texas, USA) was made close to the iris lesion. A viscoelastic was injected to fill the anterior chamber. A Kelly Descemet's membrane punch (Katena Products, Denville, New Jersey, USA; fig 1) with a 1.0 mm diameter head and a 0.75 mm deep bite was inserted into the anterior chamber to lie over the iris lesion (fig 2). The Kelly punch was placed with its mouth over the lesion and pressed down firmly before the punch was made. After taking a punch, the Kelly punch was kept closed and removed from the eye to be opened over a dry cellulose sponge (Cellulose Surgical Spear, Ivalon, San Diego, California, USA; fig 3). The viscoelastic was left in the anterior chamber.
Figure 1 A Kelly Descemet's membrane punch with a 1.0 mm diameter head and a 0.75 mm deep bite. The drawing shows a magnification of the bite tip of the tool.
Figure 2 The left eye of patient 1 with the Kelly Punch over the amelanotic iris tumour at 6 o'clock.
Figure 3 A tissue sample from patient 1 taken by a Kelly Punch biopsy over a standard dry cellulose sponge.
Pathology
The specimens were fixed in 4% formalin, routinely processed and embedded in paraffin wax. Tissue sections of thickness 4 µm were prepared and stained with H&E and S‐100, MART‐1 (melanoma antigen recognised by T cells) and cytokeratin immunostainings.
Results
Case 1
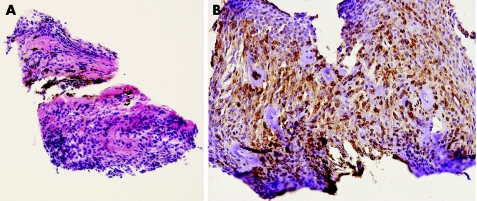
A 68‐year‐old woman presented with a vascularised non‐pigmented elevation of the peripheral inferior iris of her left eye at a routine follow‐up visit 5 years after trabeculectomy with a bleb at 12:00. The lens showed a mature cataract. Since the clinical and ultrasonic appearance was atypical for uveal melanoma of the iris and ciliary body, the patient was suspected to have a metastasis of a systemic malignancy. After general examination proved negative, an FNAB was taken. However, it did not yield enough cells to make a diagnosis. The patient underwent a biopsy using the punch biopsy technique, yielding a 0.8×0.6 mm piece of tissue (fig 3). Although the viscoelastic was not removed from the eye at the end of the surgery, the intraocular pressure (IOP) as measured postoperatively was 15 mm Hg. Tissue sections stained with H&E showed iris melanoma of the spindle B cell type, and immunostainings, positive for MART‐1 and S‐100 and negative for cytokeratin, confirmed the diagnosis of iridociliary melanoma (fig 4).
Figure 4 (A) Low‐magnification histological section showing a spindle cell melanoma (H&E, original magnification ×10). (B) Histological section with MART‐1 (melanoma antigen recognised by T cells) showing positive immunostaining of the tumour cells (original magnification ×20).
Case 2
A 63‐year‐old man with medically treated glaucoma had a pigmented lesion on the inferonasal iris of his right eye. The lesion remained stable for 20 years, but in recent years an elevation was noted growing from the centre of the pigmented area. He underwent a punch biopsy because of suspected melanoma. H&E stainings showed iris melanoma of the spindle cell type. Immunostainings were positive for MART‐1 and S‐100 and negative for cytokeratin, confirming the diagnosis. The IOP rose to 44 mm Hg postoperatively, and decreased to normal with additional topical pressure‐lowering treatment.
Discussion
In two patients with iris lesions in whom the diagnosis was uncertain, by using the punch biopsy technique with the Kelly punch we were able to obtain a piece of tissue large enough for standard histological analysis and special stainings. In both cases, H&E staining showed melanoma of spindle cell type. Tissue sections stained positive with MART‐1 and S‐100 and negative with cytokeratin, which ruled out the suspected metastatic lesion, thus establishing the diagnosis of melanoma of the iris.
In both cases, there was some bleeding from the biopsy site tamponaded by the viscoelastic filling the chamber, the wound self‐sealed and no other perioperative or postoperative complication was noted, apart from temporary IOP increase in patient 2.
The well‐known FNAB technique is excellent for aspirating cells floating in the anterior chamber, but when used on solid tumours it leads to cytopathology rather than a real tissue biopsy specimen. The modification of the aspiration technique, in which the aspiration needle is replaced by a vitrectomy probe, can provide small pieces of tissue; however, it presents several technical difficulties such as receiving the specimens in a large volume of liquid and the use of expensive equipment. The Kelly punch technique described here yields a rather large piece of tissue suitable for any type of histopathology. This technique is useful for cases in which FNAB is not diagnostic.
The Kelly punch can be inserted via a self‐sealing incision through the cornea up to a distance of 7 mm; thus, the corneal entrance site must be adjacent to the biopsy site. This 7 mm restriction poses no limitations to taking a biopsy specimen from the entire anterior surface of the iris, including the nasal iris and even the iris border, for both patients with phakia and pseudophakia. Moreover, one may even be able to take a biopsy from the posterior surface of the iris of patients with pseudophakia and aphakia.
In summary, iris biopsy specimen with the punch technique yields a tissue biopsy specimen as opposed to cytology samples obtained by FNAB. The technique is quick and safe. It is simple to perform, and, as it utilises a commonly used and easily available surgical tool, it requires no special training and is not expensive. The main benefit of using the punch biopsy technique is that the tissue obtained is of sufficient quality and quantity to enable special stainings, in addition to routine stains, which can lead to a definite diagnosis.
Abbreviations
FNAB - fine needle aspiration biopsy
IOP - intraocular pressure
MART‐1 - melanoma antigen recognised by T cells
Footnotes
Competing interests: None.
Informed consent was obtained for publication of the patients' details in this report.
References
- 1.Czerniak B, Woyke S, Domagala W.et al Fine needle aspiration cytology of intraocular malignant melanoma. Acta Cytol 198327157–165. [PubMed] [Google Scholar]
- 2.Char D H, Crawford J B, Gonzales J.et al Iris melanoma with increased intraocular pressure. Differentiation of focal solitary tumors from diffuse or multiple tumors. Arch Ophthalmol 1989107548–551. [DOI] [PubMed] [Google Scholar]
- 3.Grossniklaus H E, Brown R H, Stulting R D.et al Iris melanoma seeding through a trabeculectomy site. Arch Ophthalmol 19901081287–1290. [DOI] [PubMed] [Google Scholar]
- 4.Grossniklaus H E. Fine‐needle aspiration biopsy of the iris. Arch Ophthalmol 1992110969–976. [DOI] [PubMed] [Google Scholar]
- 5.Wakely P E, Jr, Frable W J, Geisinger K R. Aspiration cytopathology of malignant melanoma in children. A morphologic spectrum. Am J Clin Pathol 1995103231–234. [DOI] [PubMed] [Google Scholar]
- 6.Bechrakis N E, Foerster M H, Bornfeld N. Biopsy in indeterminate intraocular tumors. Ophthalmology 2002109235–242. [DOI] [PubMed] [Google Scholar]
- 7.Finger P T, Latkany P, Kurli M.et al The Finger iridectomy technique: small incision biopsy of anterior segment tumours. Br J Ophthalmol 200589946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]