Abstract
Background
Neuroglobin is a neurone specific respiratory protein that reversibly binds oxygen. Neuroglobin was discovered in 2000, initially in brain and later, at a 100 times greater concentration, in mouse retina. This protein may be involved in oxygen transport, and/or protection against oxidative stress or premature apoptosis.
Aim
To examine the expression of neuroglobin in normal human retina and also in retina from eyes with advanced glaucoma, where hypoxia and ischaemia may be pathological factors.
Methods
Immunofluorescence and electron microscopy were used to examine sections of normal human retina and retina from eyes with end‐stage glaucoma.
Results
Staining for neuroglobin was present in the plexiform layers and the photoreceptor inner segments in human retina, and increased expression was found to occur in these areas, as well as in the nuclear layers in advanced glaucoma. Much less staining for neuroglobin was present in the other retinal layers.
Conclusion
Neuroglobin is found in those layers of the human retina that are rich in mitochondria and/or synapses, and consume the highest amount of oxygen. Neuroglobin may be involved in oxygen supply to mitochondria, or in protection from oxidative stress or apoptosis. Neuroglobin expression is increased in advanced glaucoma, and it may protect against hypoxic, ischaemic or oxidative stress, which are thought to be pathological factors that affect the retina in glaucoma.
Neuroglobin is a recently discovered, mammalian, neurone specific, respiratory protein that reversibly binds oxygen and is related to haemoglobin and myoglobin.1,2,3 The function of this protein is uncertain at present, although neuroglobin may supply oxygen to the retina, functioning similarly to myoglobin in muscle.2 Another proposed function for neuroglobin is as a scavenger of reactive oxidising species.4,5 In mouse neurones, neuroglobin expression is specifically upregulated by hypoxia and ischaemia, and it may function as a neuroprotectant.6,7,8 Neuroglobin is present at a concentration that is 100 times greater in retina than in brain, accounting for 2–4% of all mouse retinal protein.2
There is evidence that the outer retina including the photoreceptor layer is under stress in glaucoma and that hypoxia and/or relative ischaemia may induce this stress.9,10,11,12,13,14,15,16 Hypoxia‐inducible factor 1α (HIF‐1α), which activates the transcription of a wide variety of genes whose protein products increase oxygen delivery or represent an adaptive response to hypoxia, is increased in glaucomatous retina, and the retinal location of this increase has been found to correlate with recorded visual field defects.9
Unlike inner retinal oxygen tension, which is maintained by autoregulation in response to increased intraocular pressure, choroidal blood flow is not regulated metabolically, and increased intraocular pressure leads to decreases in choroidal blood flow, oxygen tension and photoreceptor oxygen supply.14,15,16,17,18,19
Using immunofluorescence and electron microscopy, we examined for the presence of neuroglobin in sections of normal human retina, and searched for any changes in its expression in the retina from glaucomatous eyes where hypoxia, ischaemia and increased oxidative stress are believed to play a role.9,14,15,16,17,18,19,20
Methods
Sections of fixed human retina were obtained from the archive of the A Ray Irvine Jr, Pathology Laboratory of the Doheny Eye Institute at the University of Southern California, California, USA. Retinal sections were taken from three eyes with severe advanced chronic glaucoma, which were enucleated for pain and blindness. The diagnosis of advanced chronic glaucoma was taken from the clinical case histories and supported by histological evidence of ganglion cell degeneration in the retina and marked, deep cupping of the optic disc; the first eye was from a 4‐year old with chronic angle closure glaucoma, the second was from a 76‐year old with chronic glaucoma and a narrow angle, and the third was from a 79‐year old with chronic angle closure glaucoma and rubeosis iridis with no clinical history or histopathological sign of diabetic retinopathy. Similar age‐matched, archive sections of normal retina from three eyes without glaucoma served as controls. Appropriate negative controls were performed.
Immunostaining was carried out simultaneously on all tissue sections using an anti‐human neuroglobin antibody, which was raised against human recombinant human neuroglobin (Biovendor Laboratory Medicine, Candler, NC, USA), with an Alexa Fluor 488 fluorescent secondary antibody (Molecular Probes, Eugene, Oregon, USA). Immunofluorescent sections were viewed using a confocal microscope (Zeiss LSM 510). Electron microscopy was performed using the same primary antibody, with a gold secondary antibody.
Results
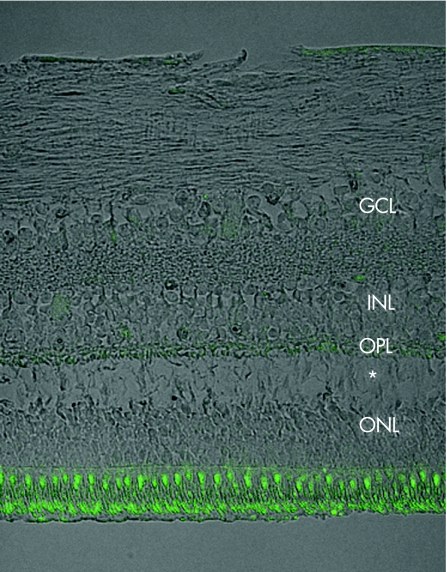
The control, normal retina revealed positive immunofluorescent staining for neuroglobin in the inner segments of the photoreceptor layer, with milder staining in the outer plexiform layer, and lesser staining in the inner plexiform and ganglion cell layers (figs 1 and 2A).
Figure 1 Immunostaining with anti‐neuroglobin antibody (green), combined with phase‐contrast image (grey) of a control retina. Staining is prominent in the photoreceptor layer, with a small amount in the outer plexiform layer (OPL). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. *Artefactual separation of the ONL and OPL.
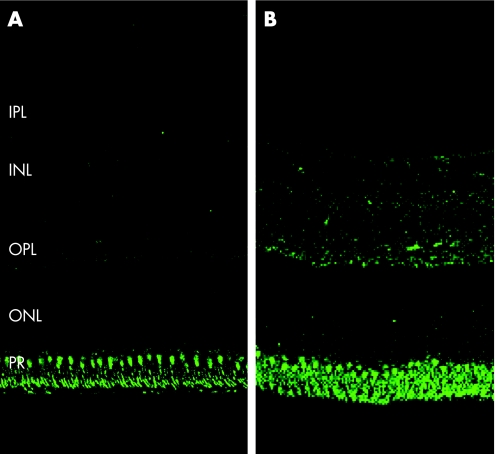
Figure 2 (A) Control (normal) retina—immunostaining with anti‐neuroglobin antibody. Staining is present in the photoreceptor and outer plexiform layers, and is minimal in the inner plexiform, nuclear and ganglion cell layers. (B) Glaucomatous retina—increased staining is noted in the inner and outer nuclear and plexiform layers, as well as in the photoreceptor layer when compared with the control, and staining remains minimal in the ganglion cell layer.
In the retina from the eyes with chronic severe glaucoma, increased intensity of neuroglobin staining was present in the photoreceptor layer, the outer and inner plexiform layers, and in addition in the outer and inner nuclear layers (fig 2B), when compared with the normal retina from the control eyes (fig 2A). Staining of the ganglion cell and nerve fibre layers was sparse in both the glaucomatous and control retina.
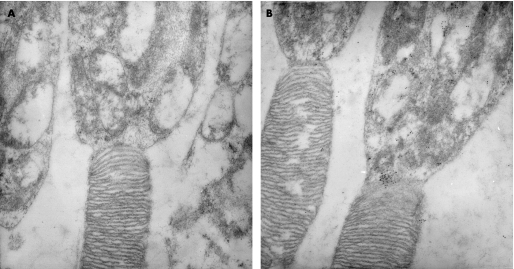
Electron microscopic examination showed much stronger staining for neuroglobin in the photoreceptor inner segments of the glaucomatous retina (fig 3B) than in the same region of the control retina. The immunogold particles were clustered in focal groups in some areas, and were most concentrated in the apical region of the inner segments (fig 3A).
Figure 3 Electron micrograph showing the increased deposits of gold label in the photoreceptor inner segments of the glaucomatous retina (B), when compared with the same region in the control retina (A); immunogold staining).
No staining was seen in the appropriate negative control experiments.
Discussion
Neuroglobin belongs to a globin lineage that is over 600 million years old, yet its existence was revealed in 2000.1,21 The sequence conservation of this nerve globin during mammalian evolution is especially high, implying a highly selected and essential function.21,22
This preliminary study demonstrates that neuroglobin is present in photoreceptors, particularly in the inner segments, as well as in the outer plexiform layer in normal human retina, with a lesser distribution in the inner plexiform and ganglion cell layers (figs 1 and 2A). The specialised architecture of the photoreceptor cell compartments and of the retina itself allows us to observe that the distribution of neuroglobin correlates with the subcellular and retinal location of mitochondria. This distribution of neuroglobin in the mitochondria and synapse‐rich, high oxygen‐consuming, inner segment and plexiform layers is found in the vascular retina of other species.2,21 In the avascular guinea pig retina, however, both mitochondria and neuroglobin are restricted in distribution to the photoreceptor inner segments, which is the only retinal layer to feature oxidative metabolism in that species. This finding, along with an oxygen affinity similar to that of myoglobin, supports the proposed role of neuroglobin in the supply of oxygen to mitochondria.2,21,23,24 Alternate putative roles for neuroglobin include; protection from reactive oxygen species or prevention of apoptosis initiation by reducing cytochrome C leakage from mitochondria.4,5,8
Oxygen consumption in the vascularised retina, as found in rats and humans, exhibits marked heterogeneity. The dominant oxygen‐consuming layers are the inner segments of the photoreceptors, the outer plexiform layer, and the deeper region of the inner plexiform layer. Relatively low oxygen consumption is a feature of the photoreceptor outer segments, the inner and outer nuclear layers and possibly the ganglion cell bodies.23 Heterogeneity has also been demonstrated in the vascular response to increased intraocular pressure: whereas oxygen tension in the inner retina is relatively unaffected by increased intraocular pressure, owing to effective oxygen autoregulation of the retinal circulation, choroidal blood flow is not subject to similar autoregulation, and increased intraocular pressure leads to reduced short posterior ciliary artery and choroidal blood flow, as well as reduced choroidal oxygen tension and photoreceptor oxygen consumption.14,15,16,17,18 This may be associated with the pathological changes demonstrated in eye bank eyes with advanced glaucoma, which include reduced choroidal thickness, decreased density of the capillaries of the choriocapillaris and of the large choroidal vessels, as well as swelling and loss of photoreceptors.10,12,13,16 Possible consequences of hypoxic or oxidative stress in the outer retina in glaucoma include the increased expression of HIF1α and heat‐shock proteins.9,11,20
In advanced chronic glaucoma, an increased amount of neuroglobin protein in the inner and outer plexiform layers, and in the photoreceptor inner segments, may be a compensatory mechanism relating to neuroglobin's proposed respiratory or neuroprotectant functions under conditions of hypoxia and/or increased oxidative stress. The relatively sparse staining for neuroglobin in the ganglion cell layer of both the normal and glaucomatous retina may indicate this protein's lack of utility in a region that is relatively unaffected by changes in oxygen tension in response to increased intraocular pressure due to effective autoregulation.
Interestingly, HIF1α, an oxygen‐regulated transcriptional activator, which activates the transcription of a wide variety of genes whose products increase oxygen delivery or represent an adaptive response to hypoxia, is increased in glaucomatous retina in locations that are closely concordant with visual field defects, and regions of HIF1α induction are believed to represent areas of decreased blood flow and hypoxia.9,20
The distribution of neuroglobin protein that we find in the normal human retina is the same as that described by Schmidt et al2 in the mouse, being almost exclusively restricted to the photoreceptor inner segments and plexiform layers. The authors also found that in contrast with the protein, the mRNA was present mainly in the large cell bodies of the nuclear and ganglion cell layers, and in the photoreceptor inner segments. This redistribution of the protein is thought to represent massive transport of neuroglobin after translation. It may be of relevance that in the glaucomatous human retina, we found that in addition to an increase in neuroglobin in the photoreceptor inner segments and the plexiform layers, the protein also appeared across both the outer and inner nuclear layers.
The increase in the presence of neuroglobin relative to the control, as demonstrated by the positive immunogold staining in the mitochondria‐rich, photoreceptor inner segments, on electron microscopy (fig 3), also suggests the presence of hypoxia or increased oxidative stress within the photoreceptor inner segments in advanced human glaucoma.
The differences in the pattern of neuroglobin expression were seen in each of the groups of age‐matched glaucomatous and control retina, which implies that the observed changes in neuroglobin expression were not age related.
It is surprising that a clear role for neuroglobin is yet to be elucidated, given that it is present in high concentration in mitochondria‐rich regions of the retina, and that other members of the globin family, haemoglobin and myoglobin, have such important and well‐described functions and properties. Further studies are required to evaluate the significance of neuroglobin in the retina and its increased presence in the retina of glaucomatous eyes.
Acknowledgements
We thank Anthony Rodriguez, Xiaopeng Wang and Monica Evans for their expertise in microscopy and immunohistochemistry, and to Professor Phil Luthert for his assistance with manuscript preparation.
Abbreviations
HIF1α - hypoxia‐inducible factor 1α
Footnotes
Competing interests: None declared.
References
- 1.Burmester T, Weich B, Reinhardt S.et al A vertebrate globin expressed in the brain. Nature 2000407520–523. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt M, Giessl A, Laufs T.et al How does the eye breathe? Evidence for neuroglobin‐mediated oxygen supply in the mammalian retina. J Biol Chem 20032781932–1935. [DOI] [PubMed] [Google Scholar]
- 3.Ostojic J, Sakaguchi D S, de Lathouder Y.et al Neuroglobin and cytoglobin: oxygen‐binding proteins in retinal neurons. Invest Ophthalmol Vis Sci 2006471016–1023. [DOI] [PubMed] [Google Scholar]
- 4.Herold S, Fago A, Weber R E.et al Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem 200427922841–22847. [DOI] [PubMed] [Google Scholar]
- 5.Herold S, Fago A. Reactions of peroxynitrite with globin proteins and their possible physiological role. Comp Biochem Physiol—A 2005142124–129. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Jin K, Mao X O.et al Neuroglobin is up‐regulated by and protects neurons from hypoxic‐ischemic injury. Proc Natl Acad Sci USA 20019815306–15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Jin K, Peel A.et al Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA 20031003497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fago A, Mathews A J, Moens L.et al The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett 20065804884–4888. [DOI] [PubMed] [Google Scholar]
- 9.Tezel G, Wax M. Hypoxia‐inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol 20041221348–1356. [DOI] [PubMed] [Google Scholar]
- 10.Nork T M, Ver Hoeve J N, Poulsen G L.et al Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol 2000118235–245. [DOI] [PubMed] [Google Scholar]
- 11.Tezel G, Hernandez M R, Wax M B. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol 2000118511–518. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, Jonas J B. Decreased photoreceptor count in human eyes with secondary angle‐closure glaucoma. Invest Ophthalmol Vis Sci 1992332532–2536. [PubMed] [Google Scholar]
- 13.Kubota T, Jonas J B, Naumann G O. Decreased choroidal thickness in eyes with secondary angle closure glaucoma. An aetiological factor for deep retinal changes in glaucoma? Br J Ophthalmol 199377430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joos K M, Kay M D, Pillunat L E.et al Effect of acute intraocular pressure changes on short posterior ciliary artery haemodynamics. Br J Ophthalmol 19998333–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duijm H F A, van den Berg T J T P, Greve E L. Choroidal haemodynamics in glaucoma. Br J Ophthalmol 199781735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spraul C W, Lang G E, Lang G K.et al Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vis Res 200242923–932. [DOI] [PubMed] [Google Scholar]
- 17.Yancey C, Linsenmeier R. Oxygen distribution and consumption in the cat retina at increased intraocular pressure. Invest Ophthalmol Vis Sci 198930600–611. [PubMed] [Google Scholar]
- 18.Wangsa‐Wirawan N D, Linsenmeier R A. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 2003121547–557. [DOI] [PubMed] [Google Scholar]
- 19.Alder V A, Cringle S J. Intraretinal and preretinal PO2 response to acutely raised intraocular pressure in cats. Am J Physiol Heart Circ Physiol 1989256H1627–H1634. [DOI] [PubMed] [Google Scholar]
- 20.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 200625490–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankeln T, Ebner B, Fuchs C.et al Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J Inorg Biochem Part 1 200599110–119. [DOI] [PubMed] [Google Scholar]
- 22.Wystub S, Ebner B, Fuchs C.et al Interspecies comparison of neuroglobin, cytoglobin and myoglobin: sequence evolution and candidate regulatory elements. Cytogenet Genome Res 200410565–78. [DOI] [PubMed] [Google Scholar]
- 23.Yu D ‐ Y, Cringle S J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 200120175–208. [DOI] [PubMed] [Google Scholar]
- 24.Bentmann A, Schmidt M, Reuss S.et al Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem 200528020660–20665. [DOI] [PubMed] [Google Scholar]