Retinitis pigmentosa is a genetically heterogeneous group of progressive retinal degenerations.1 Autosomal recessive retinitis pigmentosa caused by mutations in the gene encoding the β‐subunit of rod photoreceptor cyclic guanosine monophosphate‐phosphodiesterase (PDE6B) was one of the first forms to be identified, and there are well‐studied murine and canine animal models as well as proof‐of‐concept success of somatic gene therapy.1,2,3,4 Rapid rod photoreceptor degeneration in the animal models is complicated by morphological changes involving the inner retina.5,6 It is unknown, however, whether the human form of retinitis pigmentosa is also complicated by retinal remodelling; the answer could have implications for treatment potential. We used optical coherence tomography (OCT) to study the retina of a patient with retinitis pigmentosa with a known PDE6B null mutation,7 and found there was abnormal laminar architecture suggesting retinal remodelling.
Case report
A 25‐year‐old woman with retinitis pigmentosa was homozygous for the Cys270X mutation in PDE6B. There was no rod function and only severely impaired cone function.7
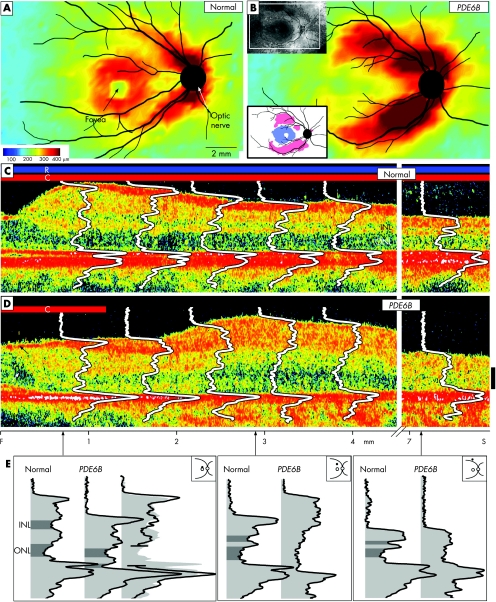
At 34 years of age, her best‐corrected visual acuity remained 20/30, but visual fields had decreased to only a central island. OCT was performed using topographical mapping and longitudinal reflectivity profile (LRP) analyses.8 Retinal thickness topography in the patient differed dramatically from normal (fig 1A). Especially notable was the abnormally thickened retina along the arcades (fig 1B). A difference map highlights parafoveal thinning and patches of thickened superior and inferior retina (fig 1B, inset). Laminar architecture was explored using LRPs overlaid on cross‐sectional images from the fovea into the superior retina (fig 1C,D). The patient had laminated but thinned retina in the parafovea, and, with increasing eccentricity, there was a coarsely laminated and thickened region. At further superior loci, the retina had normal thickness but was delaminated. A more detailed comparison was made of LRPs at three eccentricities (fig 1E). At the parafoveal locus, thinning could be accounted for by missing retinal layers, specifically loss of photoreceptor waveform components. At the more superior loci, whether increased in thickness or not, the patient's retina had no comparable lamination with normal retinal.
Figure 1 (A,B) Retinal topography in a normal subject (26 years of age) and in a patient with retinitis pigmentosa with PDE6B mutations (34 years of age). Insets in (B): upper left, fundus view and window where optical coherence tomography (OCT) mapping was performed; lower left, difference map from normal thickness (n = 5, ages 21–26 years). White represents within normal limits (±2SD); blue represents below and pink above normal limits. Fundus landmarks of the optic nerve and major retinal vessels are drawn on the maps. Cross‐sectional OCT images with overlaid longitudinal reflectivity profiles (LRPs) from the fovea into the superior retina of a normal subject (C; aged 28 years) and of the patient (D; arrows points to cystoid oedema, which was also evident on ophthalmoscopy, and epiretinal membrane). Bars above the cross‐sections indicate presence or absence of rod (R) and cone (C) function, measured by dark‐adapted perimetry. The patient has no rod function and detectable cone function in the central field only. Calibration bar at right: 100 μm. (E) Reflectivity profiles from three loci (0.7, 2.9 and 7.5 mm superior) to illustrate the differences between the patient's laminae and those of the normal subject. At the 0.7 mm locus, the patient profile is split and the two parts overlaid on the representative normal profile (third profile at the right in this subpanel). This is to test the hypothesis that lost photoreceptor components explain the thinness of the retina. At 2.9 and 7.5 mm superior loci, the abnormal lamination in the patient profile precludes testing the hypothesis about missing components. F, fovea; INL, inner nuclear layer; ONL, outer nuclear layer; S, superior.
Comment
The OCT results in this patient with retinitis pigmentosa and PDE6B mutations are complex but interpretable. Parafoveal thinning is attributable to rod (and cone) photoreceptor layer losses. The remarkable thickening and loss of normal laminar pattern at further eccentricities is probably an OCT marker for retinal disorganisation. Thickened and dysplastic‐appearing retina on OCT scans has been previously reported in two early‐onset retinal degenerations with a developmental component: one, a form of Leber congenital amaurosis caused by CRB1 mutations,8 and the other, enhanced S cone syndrome due to NR2E3 mutations.9 The present observations are the first in a form of retinitis pigmentosa. We propose that the results represent in vivo evidence for retinal remodelling, a process involving neuronal loss and migration, glial hypertrophy and aberrant circuitry occurring in reaction to photoreceptor death. Retinal remodelling has been demonstrated using histopathology in postmortem human retinas and in animals with retinal degeneration,10 including those with PDE6B mutations.5,6 Identifying retinal remodelling in human retinal degenerations will be valuable in future clinical trials as a structural criterion to determine the potential for therapeutic benefit.
Acknowledgements
This study was supported by the Foundation Fighting Blindness, Macula Vision Research Foundation, Macular Disease Foundation, Ruth and Milton Steinbach Fund, Alcon Research Institute, Mackall Foundation and the FM Kirby Foundation. We thank Elizabeth Windsor, Alejandro Roman and Malgorzata Swider for help with data collection and analyses.
Footnotes
Competing interests: None declared.
References
- 1.Kalloniatis M, Fletcher E L. Retinitis pigmentosa: understanding the clinical presentation, mechanisms and treatment options. Clin Exp Optom 20048765–80. [DOI] [PubMed] [Google Scholar]
- 2.Bowes C, Li T, Danciger M.et al Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP‐phosphodiesterase. Nature 1990347677–680. [DOI] [PubMed] [Google Scholar]
- 3.Farber D B, Danciger J S, Aguirre G. The beta subunit of cyclic GMP phosphodiesterase mRNA is deficient in canine rod‐cone dysplasia 1. Neuron 19929349–356. [DOI] [PubMed] [Google Scholar]
- 4.Pang J, Cheng M, Stevenson D.et al Adenoviral‐mediated gene transfer to retinal explants during development and degeneration. Exp Eye Res 200479189–201. [DOI] [PubMed] [Google Scholar]
- 5.Strettoi E, Porciatti V, Falsini B.et al Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci 2002225492–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeiss C J, Allore H G, Towle V.et al CNTF induces dose‐dependent alterations in retinal morphology in normal and rcd‐1 canine retina. Exp Eye Res 200682395–404. [DOI] [PubMed] [Google Scholar]
- 7.Danciger M, Heilbron V, Gao Y Q.et al A homozygous PDE6B mutation in a family with autosomal recessive retinitis pigmentosa. Mol Vis 1996210. [PubMed] [Google Scholar]
- 8.Jacobson S G, Cideciyan A V, Aleman T S.et al Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet 2003121073–1078. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson S G, Sumaroka A, Aleman T S.et al Nuclear receptor NR2E3 gene mutations distort human retinal laminar architecture and cause an unusual retinal degeneration. Hum Mol Genet 2004131893–1902. [DOI] [PubMed] [Google Scholar]
- 10.Marc R E, Jones B W, Watt C B.et al Neural remodelling in retinal degeneration. Prog Retin Eye Res 200322607–655. [DOI] [PubMed] [Google Scholar]