Abstract
Background
Ocular involvement of syphilis still poses a clinical challenge due to the chameleonic behaviour of the disease. As the serodiagnosis has significant limitations, the direct detection of Treponema pallidum (TP) in the vitreous represents a desirable diagnostic tool.
Methods
Real‐time polymerase chain reaction (PCR) for the detection of TP was applied in diagnostic vitrectomies of two patients with acute chorioretinitis. Qualitative verification of TP by real‐time PCR and melting point analysis according to a modified protocol was ruled out. Patients underwent complete ophthalmological examination with fundus photographs, fluorescein angiography, serological examination, antibiotic treatment and follow‐up.
Results
In two cases of acute chorioretinitis of unknown origin, real‐time PCR of vitreous specimens of both patients provided evidence of TP and was 100% specific. Initial diagnosis of presumed viral retinitis was ruled out by PCR of vitreous specimen. Patients were treated with systemic antibiotics and showed prompt improvement in visual function and resolution of fundus lesions.
Conclusions
With real‐time PCR, detection of TP in the vitreous was possible and delivered a sensitive, quick and inexpensive answer to a disease rather difficult to assess. In cases of acute chorioretinitis, the use of PCR‐based assays of vitreous specimens in the diagnostic evaluation of patients is advisable. Although syphilitic chorioretinitis is a rare disease, PCR should include search for TP, as diagnostic dilemmas prolong definitive treatment in a sight‐threatening disease.
Ocular involvement of syphilis still represents a rare and important eye disease and may occur in different clinical manifestations.1,2,3,4 It is burdened with difficult diagnosis and detection of the causative pathogen Treponema pallidum (TP).5,6,7 TP was so far detected by polymerase chain reaction (PCR) in different human specimens.8,9,10,11,12 Although there is evidence of TP in aqueous humour,13 to our knowledge no direct reference of the bacterium in vitreous tap has been successful. In this non‐comparative interventional case series, we report on two patients in whom the causative pathogen was TP and could be established by PCR.
Methods
PCR analysis
TP was detected qualitatively by PCR and melting point analysis. Extraction protocol using a silica spin column (Roche Molecular Biochemicals, Mannheim, Germany) was modified according to earlier published methods7,11,14 of the National Reference Center for Treponema pallidum (Laboratory Krone and partners, Bad Salzuflen, Germany). In brief, specimens were incubated in reaction buffer at 56°C. The samples were mixed with lysis buffer and heated up to 95°C. After controlled cooling, selective DNA binding was performed on a silica matrix using a spin column. During amplification of organism‐specific DNA with PCR, the amplification products underwent fluorescence detection with SYBR green dye (Invitrogen, Carlsbad, California, USA) with the LightCycler (Roche Molecular Biochemicals). Amplification of the specific fragment was confirmed by analysis of the melting temperature of the amplified DNA fragment. All samples were analysed in duplicate PCR runs. For quality control, 1 μl of each reaction mix was analysed in the Bioanalyser instrument (Agilent, Palo Alto, California, USA) using the LabChip 7500 kit to visualise the expected product of 260 bp.
Patients
Two patients underwent complete ophthalmological examination with fundus photographs, fluorescein angiography and serological examination. Vitreous biopsy specimens, using the initial preinfusion aspirate (100–500 μl), were collected and sent for culture, cytological and PCR analysis.
Results
The PCR of vitreous tap for both patients provided evidence of TP. For high specific amplification of TP, primer sequences were chosen for DNA of the 47 kDa protein. To evaluate the specificity and sensitivity of the protocol, real‐time PCR was performed using TP‐positive homogenates of rabbit testis (3+) in serial dilutions. All TP controls were detected when diluted 10‐fold, 100‐fold and 1000‐fold with 100% sensitivity, representing a detection limit of an estimated number of 100 organisms/ml. This corresponds to an equivalent of 1 IFU per PCR assay. In a preceding pilot study, 8 specimens out of 59 different samples gave a positive signal after 10‐fold and 100‐fold dilution, indicating a minimum titre of 104 IFU/ml in the undiluted sample. The observed melting temperature for all positive samples was the same as the expected value of about 88°C (0.5°C).
Case 1
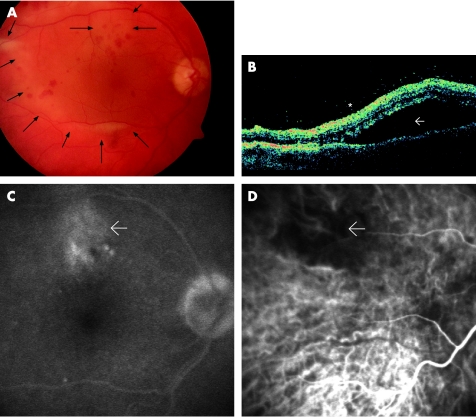
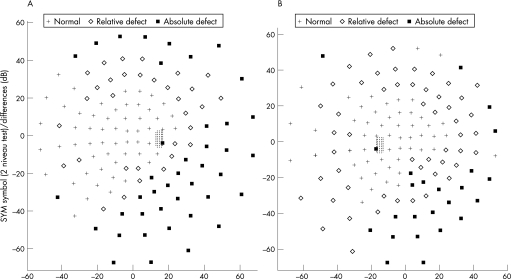
A 67‐year‐old man was referred with uveitis and inferior peripheral field deterioration of his right eye. He had already received treatment with 60 mg of oral prednisolone for 1 week without improvement. Visual acuity (VA) was 0.4 in OD and 1.0 in OS. OD presented endothelial precipitates, cells 2+ and vitreous cells, and flare. Nasal‐and mid‐periphery of the retina revealed patchy, confluent, yellowish areas without haemorrhage. OS was unremarkable. A diagnostic vitrectomy was performed, and he was treated with systemic antiviral therapy (acyclovir 750 mg thrice daily intravenously) and 100 mg/day of prednisolone for 1 week, followed by tapering dosages for another week. After 2 weeks, he presented with similar lesions in the outer periphery of the left eye. Bilateral acute retinal necrosis was the presumed diagnosis, and diagnostic vitrectomy of the left eye with intravitreal injection of 2.4 mg Foscarnet was performed. As VA dropped in the right eye due to central involvement, two injections of 2.4 mg Foscarnet intravitreously were performed. Fundus of both eyes showed chorioretinitis with placoid, pale yellow lesions, intraretinal haemorrhages and serous detachment of the posterior pole (fig 1A). VA accounted for 0.1 in each eye at that stage. Figs 1 and 2 show the clinical features. Serological testing was unremarkable for acute infection or reactivation of cytomegalovirus (CMV), varicella zoster virus, herpes simplex virus (HSV), human immune deficiency virus (HIV), hepatitis C virus and toxoplasmosis. Vitreous taps were tested negative for direct detection of viral DNA. The patient had serological evidence of having passed through hepatitis A, B and late‐stage borreliosis, as his medical history was remarkable for tick bite (borreliosis ELISA IgG positive, IgM negative, borreliosis Western blot positive, IgM negative). Because of a positive Treponema pallidum particle agglutination‐screening test, which was conducted with the borreliosis serological testing for cross‐reactivity,15 the complete syphilis serological testing was performed (table 1). At this stage, the vitreous tap revealed a positive result for TP. Further dermatological and neurological examination was unremarkable. Past venereal disease was denied and one promiscuous contact 5 years ago was stated. The patient received 2 g/day of ceftriaxon intravenously for 3 weeks. After 3 months, Treponema pallidum particle agglutination and lipoid antibody titre (cardiolipin) declined. Fluorescent treponemal antibody absorption‐19s‐IgM test declined from 1:320 to 1:20. VA recovered to OD 0.6 OD 0.8 OS, but visual fields remained burdened, with peripheral relative and absolute visual field defects.
Figure 1 Case 1. Left eye:(A) Fundus photograph showing chorioretinitis with placoid, pale, yellowish lesions, subretinal fluid accumulation (→) and intraretinal haemorrhages. (B) Optical coherence tomography revealing extensive serous detachment of the posterior pole (→) and condensed vitreous close to the retinal surface (*) (C) Late‐phase fluorescein angiography disclosed late staining at the level of the retinal pigment epithelium that was most prominent in the areas of yellowing. (D) Indocyanine green angiography showing early hypofluorescence in the same area (→).
Figure 2 Case 1. 60° automatic perimetry (Octopus 500EZ, program07, Interzeag, Gera, Germany) demonstrating compromised visual fields in the (A) Right eye and (B) Left eye
Table 1 Syphilis serological testing (in serum and cerebrospinal fluid ).
| Material | TPPA | FTA‐ABS | Cardiolipin | IgM–blot | ||
|---|---|---|---|---|---|---|
| IgG | IgM | KBR | Tpallidum | VDRL | ||
| Serum | 1:40 000 | 1:20 000 | 1:320 | 100 IU/ml | positive | positive |
| CSF | 1:512 | 1:256 | Undetermined | 4 IU/ml | negative | positive |
CSF, cerbrospinal fluid; FTA‐ABS, fluorescent treponemal antibody absorption; KBR, potassium bromide; VDRL, Veneral Disease Research Laboratory; TPPA, Treponema pallidum particle agglutination.
Case 2
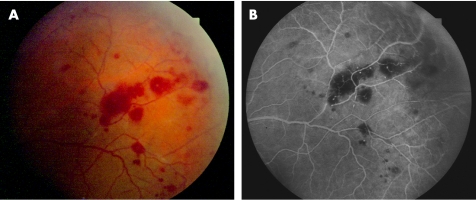
A 43‐year‐old Hispanic man was referred to the department of internal medicine with blurred vision. He was diagnosed with abdominal lymphoma of unknown origin and splenomegaly. He was HIV‐negative and HBV‐positive with a CD4 count of 500/μl. VA was OD 0.4 and OS 0.5. He presented with endothelial precipitates, cells 2+ and flare in the anterior chamber, and dense condensations of the vitreous. The central retina showed massive yellowish infiltrates and some sheathing of the retinal vessels, with intraretinal haemorrhages. He was presumed to have cytomegalovirus retinitis and was treated with valaciclovir and ceftriaxon intravenously and prednisone 50 mg orally. Diagnostic vitrectomy was performed on OS. The PCR of the vitreous tap revealed a positive result for TP and was tested negative for HSV, varicella zoster virus, HSV type 1 and 2, CMV, Epstein–Barr Virus (EBV), Human Herpes Virus type 6, 7, 8 (HHV) and mycobacteria. The latter was negative in culture and microscopy, but Pseudomonas aeruginosa was isolated. After treatment with ceftriaxon for 4 weeks, VA was restored to OD 0.6 and OS 0.8. At 2 weeks after onset oftreatment, no inflammatory sign was found any more in the vitreous. Fluorescein angiography revealed arteriolar occlusion inferotemporally (fig 3).
Figure 3 Case 2. Left eye: (A) Fundus photograph 2 weeks after onset of treatment with residual intraretinal haemorrhages and vascular sheathing. (B) Late‐phase fluorescein angiography indicating retinal arteriolar occlusion inferotemporally.
The diagnosis of multicentric Morbus Castleman disease was established by laparoscopic lymphadenectomy. This is a rare lymphoproliferative disease associated with HHV 8 and HIV, and a body‐cavity lymphoma (high malignant non‐Hodgkin's‐lymphoma). The patient was treated with antiretroviral therapy and polychemotherapy (rituximab+cyclophosphamide+doxorubicin+vincristine+prednisone) and died 6 months later.
Discussion
The mimicry of syphilis with several other inflammatory ocular diseases may lead to misdiagnosis and delays in appropriate antimicrobial treatment, like in the presented patients, who were misdiagnosed with bilateral acute retinal necrosis and CMV retinitis, respectively, and treated with antiviral therapy. In the absence of culture of TP, a nested PCR to amplify a specific segment of the genome is valuable and was performed by Pietravalle et al.16 They emphasised the diagnostic relevance for TP in different phases of infection and found positive results in ulcerative secretions and in sera. In fact, even after treatment, ulcerative secretions that were negative in dark‐field examination were found to be positive in PCR. Grimprel et al14 demonstrated 100% sensitivity compared with rabbit infectivity testing in detecting TP in amnionic fluid of congenital syphilis. Liu et al10 tested DNA polymerase I gene (pol A) and obtained a sensitivity of 95.8% and a specificity of 95.7% in 112 genital ulcers, and suggest that the polA PCR is applicable as a routine clinical diagnostic test for syphilis. Zoechling et al17 used the highly sensitive technique of PCR in late secondary and tertiary skin lesions. The detection of low numbers of TP in clinical materials is difficult.18 However, our approach of the described DNA technique revealed extremely high sensitivity and specificity.19 Compared with conventional PCR, the real‐time PCR technique described here proves to be both ultra‐rapid and sensitive in allowing detection in less than 3 h.
Table 2 Protein parameter in serum and cerebrospinal fluid.
| Result | Dimension | Reference range | |
|---|---|---|---|
| Albumin in serum | 4.07 | g/dl | 3.6–6.8 |
| Total‐IgG in serum | 818.0 | mg/dl | 700–1600 |
| Total‐IgM in serum | 29.0 | mg/dl | 40–230 |
| Albumin in CSF | 24.7 | mg/dl | 18–33 |
| Total‐IgG in CSF | 3.8 | mg/dl | 1.4–3.5 |
| Total‐IgM in CSF | 0.53 | mg/dl | <0.1 |
CSF, cerebrospinal fluid.
Table 3 Cerebrospinal fluid ‐serum‐ratio.
| Result | Reference | |
|---|---|---|
| Albumin ratio | 6.1 | 5–8 |
| TPPA ratio | 2 | <4 |
| FTA‐ABS—IgG–ratio | 2.9 | <4 |
TPPA, treponema pallidum particle agglutination.
Our case 2 surprisingly also revealed P aeruginosa in the culture of the vitreous tap. However, the prolonged history and typical behaviour of P aeruginosa causing a devastating acute endophthalmitis argue against a clinical correlation of this finding and suggest a secondary contamination during handling of the diagnostic specimen.
As demonstrated, the detection of TP in vitreous biopsy allowed definitive diagnosis and adequate treatment in a sight‐threatening infection during active disease. Therefore, we advocate TP‐PCR as a highly useful diagnostic procedure. Although the most common causes of chorioretinitis are viral agents, in macula impending circumstances a PCR of TP is recommended within the first analysis. In all other cases of peripheral involvement, due to cost reduction, PCR of TP should ultimately be considered after a negative viral PCR. The results of the current study further demonstrate the importance and utility of vitreous biopsy as a diagnostic procedure for a selected group of patients in which diagnostic clinical patterns of ocular inflammation may be obscured due to the chameleonic behaviour of the disease.
Abbreviations
CMV - cytomegalovirus
FTA‐ABS - fluorescent treponemal antibody absorption
HSV - herpes simplex virus
PCR - polymerase chain reaction
VA - visual acuity
References
- 1.Gass J D M, Braunstein R A, Chenoweth R G. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology 1990971288–1297. [DOI] [PubMed] [Google Scholar]
- 2.Arruga J, Valentines J, Mauri F.et al Neuroretinitis in acquired syphilis. Ophthalmology 198592262–270. [DOI] [PubMed] [Google Scholar]
- 3.Folk J C, Weingeist T A, Corbett J J.et al Syphilitic neuroretinitis. Am J Ophthalmol 198395480–486. [DOI] [PubMed] [Google Scholar]
- 4.Mora P, Borruat F ‐ X, Guex‐Crosier Y. Indocyanine green angiography anomalies in ocular syphilis. Retina 200525171–181. [DOI] [PubMed] [Google Scholar]
- 5.Aldave A J, King J A, Cunningham E T., Jr Ocular syphilis. Curr Opin Ophthalmol 200112433–441. [DOI] [PubMed] [Google Scholar]
- 6.Woznicova V, Heroldova M. Direct detection of Treponema pallidum in diagnosis of syphilis. Epidemiol Mikrobiol Immunol 200453121–125. [PubMed] [Google Scholar]
- 7.Burstain J M, Grimprel E, Lukehart S A.et al Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol 19912962–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noordhoek G T, Wolters E C, de Jonge M E.et al Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol 1991291976–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer H M, Higgins S P, Herring A J.et al Use of PCR in the diagnosis of early syphilis in the United Kingdom. Sex Transm Infect 200379479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Rodes B, Chen C Y.et al New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J Clin Microbiol 2001391941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centurion‐Lara A, Castro C, Shaffer J M.et al Detection of Treponema pallidum by a sensitive reverse transcriptase PCR. J Clin Microbiol 1997351348–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagaki H, Kawai T, Miyata M.et al Gastric syphilis: polymerase chain reaction detection of treponemal DNA in pseudolymphomatous lesions. Hum Pathol 199627761–765. [DOI] [PubMed] [Google Scholar]
- 13.Smith J L, Israel C W. Spirochetes in the aqueous humor in seronegative ocular syphilis. Persistence after penicillin therapy. Arch Ophthalmol 196777474–477. [DOI] [PubMed] [Google Scholar]
- 14.Grimprel E, Sanchez P J, Wendel G D.et al Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid, fetal and neonatal sera and cerebrospinal fluid. J Clin Microbiol 1991291711–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruckbauer H R, Preac‐Mursic V, Fuchs R.et al Cross‐reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis 19921224–232. [DOI] [PubMed] [Google Scholar]
- 16.Pietravalle M, Pimpinelli F, Maini A.et al Diagnostic relevance of polymerase chain reaction technology for T. pallidum in subjects with syphilis in different phases of infection. New Microbiol 19992299–104. [PubMed] [Google Scholar]
- 17.Zoechling N, Schluepen E M, Soyer H P.et al Molecular detection of Treponema pallidum in secondary and tertiary syphilis. Br J Dermatol 1997136683–686. [PubMed] [Google Scholar]
- 18.Radolf J D. PCR detection of treponema pallidum. In: Persing DH, et al, eds. Diagnostic molecular microbiology. Blackwell Publishing, Washington, DC 1993224–229.
- 19.Van Gelder R N. Applications of the polymerase chain reaction to diagnosis of ophthalmic disease. Surv Ophthalmol 200146248–258. [DOI] [PubMed] [Google Scholar]