Abstract
Aim
To determine the aqueous humour concentration of the acid hydrolysis products of bimatoprost and latanoprost after a single topical dose of bimatoprost 0.03% or latanoprost 0.005% in humans.
Methods
Randomised, controlled, double‐masked, prospective study. 48 eyes of 48 patients scheduled for routine cataract surgery were randomised in an 8:2:2 ratio to treatment with a single 30 μl drop of bimatoprost 0.03%, latanoprost 0.005% or placebo at 1, 3, 6 or 12 h before the scheduled cataract surgery. Aqueous humour samples were withdrawn at the beginning of the surgical procedure and analysed using high‐performance liquid chromatography–tandem mass spectrometry.
Results
Bimatoprost acid (17‐phenyl trinor prostaglandin F2α) was detected in aqueous samples at a mean concentration of 5.0 nM at hour 1, 6.7 nM at hour 3 and 1.9 nM at hour 6 after bimatoprost treatment. After latanoprost treatment, the mean concentration of latanoprost acid (13,14‐dihydro‐17‐phenyl trinor prostaglandin F2α) in aqueous samples was 29.1 nM at hour 1, 41.3 nM at hour 3 and 2.5 nM at hour 6. Acid metabolites were below the limit of quantitation in all samples taken 12 h after dosing and in all samples from placebo‐treated patients. None of the samples from latanoprost‐treated patients contained quantifiable levels of non‐metabolised latanoprost. Non‐metabolised bimatoprost was detected in aqueous samples at a mean concentration of 6.6 nM at hour 1 and 2.4 nM at hour 3 after bimatoprost treatment.
Conclusions
Low levels of bimatoprost acid were detected in aqueous humour samples from patients with cataract treated with a single dose of bimatoprost. Latanoprost acid concentrations in samples from patients treated with latanoprost were at least sixfold higher. These results suggest that bimatoprost acid in the aqueous humour does not sufficiently account for the ocular hypotensive efficacy of bimatoprost.
The intraocular pressure (IOP)‐lowering drug latanoprost is a prodrug that must be metabolised to show meaningful pharmacological activity.1 After topical application, latanoprost is rapidly hydrolysed to latanoprost acid (13,14‐dihydro‐17‐phenyl trinor prostaglandin F2α (PGF2α)), a potent prostaglandin FP receptor agonist responsible for the subsequent reduction in IOP.1
By contrast, the IOP‐lowering drug bimatoprost seems to be a drug that does not have to be metabolised to exhibit biological activity. In the living non‐human primate eye, topical treatment with bimatoprost provides substantial reductions in IOP, but the acid hydrolysis product of bimatoprost is absent or at negligible levels.2 Hydrolysis of bimatoprost also seems to be slow and inefficient in human ocular tissues in vitro.3,4
Studies of drug metabolism after a single dose of bimatoprost in humans might be informative in determining the importance of drug metabolism to the IOP lowering produced by bimatoprost, as clinical studies have shown that even a single dose of bimatoprost causes a significant reduction in IOP.5 The purpose of this study was to determine the aqueous humour concentrations of bimatoprost and 17‐phenyl trinor PGF2α (bimatoprost acid) in humans after a single topical application of bimatoprost compared with the levels of latanoprost and its acid metabolite after a single topical dose of latanoprost.
Methods
This was a randomised, vehicle‐controlled, double‐masked, prospective study conducted in compliance with the Declaration of Helsinki. All patients provided written informed consent before study entry. Eligible patients were scheduled for routine cataract removal, had no history of topical prostaglandin use and had not used drugs for glaucoma in the 6 weeks before surgery. Primary exclusion criteria included any contraindication to topical use of a prostaglandin analogue, laser or other intraocular surgery within the past 3 months, and any situation or condition that might put the patient at risk or confound the study results. Study eyes (n = 48; one eye for each patient) were randomised in an 8:2:2 ratio to treatment with a single 30 μl drop of bimatoprost 0.03%, latanoprost 0.005% or placebo by micropipette at 1, 3, 6 or 12 h before surgery.
Preoperative medications included phenylphrine 2.5% and cyclopentolate 1% administered every 5 min for a total of three doses beginning 30 min before surgery. At the initiation of surgery, a corneal paracentesis incision was made with a 30 G needle on a 1 ml tuberculin syringe, and an aqueous sample (100–200 µl) was taken. The aqueous sample was capped, labelled with the patient number, placed on dry ice and transported to a −85°C freezer for storage before analysis. The surgeons who collected the samples and the investigators who assayed the samples were blinded to the treatment.
Levels of bimatoprost, latanoprost and their acid hydrolysis products (17‐phenyl trinor PGF2α and 13,14‐dihydro‐17‐phenyl trinor PGF2α, respectively) were determined by an independent laboratory using a validated method of high‐performance liquid chromatography–tandem mass spectrometry (HPLC‐MS/MS). Tetradeuterated (d4) or pentadeuterated (d5) internal standards were used for assay validation. Calibration standards, quality control samples and study samples were prepared by adding internal standard solution (bimatoprost‐d5 and bimatoprost acid‐d4, or latanoprost‐d4 and latanoprost acid‐d4) to 100 μl of aqueous humour. After addition of 10 μl of 10% formic acid and 1 ml of methyl tert‐butyl ether, analytes were extracted into the organic phase and extracts were dried, reconstituted in HPLC mobile phase, and analysed on a Micromass Quattro LC (Waters, Milford, Massachusetts, USA) liquid chromatography/mass spectrometry system using electrospray ionisation and the multiple reaction monitoring mode for quantitation. Bimatoprost and bimatoprost acid were analysed using normal‐phase HPLC–MS/MS on an APS‐2 Hypersil (3 μm, 2.1×150 mm) column with a gradient mobile phase of acidified acetonitrile–methanol and positive‐to‐negative ion switch. Latanoprost and latanoprost acid were analysed using reverse‐phase HPLC–MS/MS on a BDS Hypersil C8 (5 μm, 4.6×50 mm) column (Thermo Scientific, Waltham, MA, USA) with a gradient mobile phase of acidified methanol–water and negative‐to‐positive ion switch. The precursor–product ion pairs used in multiple reaction monitoring analysis were (m/z) bimatoprost: 416.5(MH+)>362.3; bimatoprost acid: 387.6(M−H)−>193.0; bimatoprost‐d5: 421.5(MH+)>367.3; bimatoprost acid‐d4: 391.6(M−H)−>197.0; latanoprost: 433.5(MH+)>379.5; latanoprost acid: 389.3(M−H)−>345.5; latanoprost‐d4: 437.5(MH+)>383.5; and latanoprost acid‐d4: 393.3(M−H)−>349.5. Mean retention times were 3.1, 3.6, 3.7 and 5.5 min for latanoprost acid, latanoprost, bimatoprost and bimatoprost acid, respectively. The lower limit of quantitation was 0.5 ng/ml bimatoprost (1.2 nM), 0.5 ng/ml bimatoprost acid (1.3 nM), 1.0 ng/ml latanoprost (2.3 nM) and 1.0 ng/ml latanoprost acid (2.6 nM).
Results
Aqueous samples from all 48 randomised eyes were analysed for bimatoprost, latanoprost and their acid metabolites. Bimatoprost levels in 2 of 32 bimatoprost‐treated eyes could not be determined because of inadequate sample volumes. Acid metabolite levels were evaluated for all 48 eyes.
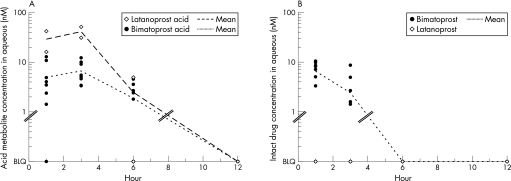
Aqueous samples taken from patients with cataract at 1, 3, 6 or 12 h after a single dose of bimatoprost contained low or undetectable levels of bimatoprost acid. Measured levels of bimatoprost acid ranged from below the limit of quantitation (BLQ) to 12.9 nM at hour 1, from 3.4 nM to 12.2 nM at hour 3 and from BLQ to 4.6 nM at hour 6 (fig 1A, table 1).
Figure 1 Levels of acid metabolite (A) and intact drug (B) in aqueous humour samples of patients with cataract after a single dose of bimatoprost or latanoprost. BLQ, below the limit of quantitation.
Table 1 Acid metabolite levels in aqueous humour samples from patients with cataract.
| Acid metabolite levels* | ||||
|---|---|---|---|---|
| Hour 1 | Hour 3 | Hour 6 | Hour 12 | |
| Bimatoprost acid after bimatoprost treatment (n = 8 at each time point) | ||||
| Mean (SD) | 5.0 (4.5) nM | 6.7 (3.3) nM | 1.9 (1.8) nM | 0 (0) nM |
| Median | 3.8 nM | 5.3 nM | 2.1 nM | 0 nM |
| Range | BLQ–12.9 nM | 3.4–12.2 nM | BLQ–4.6 nM | All samples BLQ |
| Latanoprost acid after latanoprost treatment (n = 2 at each time point) | ||||
| Mean (SD) | 29.1(13.0) nM | 41.3 (10.2) nM | 2.5 (2.5) nM | 0 (0) nM |
| Median | 29.1 nM | 41.3 nM | 2.5 nM | 0 nM |
| Range | 16.1–42.1 nM | 31.1–51.4 nM | BLQ–4.9 nM | Both samples BLQ |
BLQ, below the limit of quantitation.
*Samples with acid metabolite BLQ (limit of quantitation was 0.5 ng/ml or 1.3 nM for bimatoprost acid and 1.0 ng/ml or 2.6 nM for latanoprost acid) were assigned a value of 0 nM.
Bimatoprost acid was BLQ in three of the eight 6 h samples and in all eight of the 12 h samples. Mean (SD) levels of bimatoprost acid were 5.0 (4.5) nM at hour 1, 6.7 (3.3) nM at hour 3, 1.9 (1.8) nM at hour 6 and 0(0) nM at hour 12.
In comparison, after a single dose of latanoprost, levels of latanoprost acid in aqueous samples ranged from 16.1 to 42.1 nM, 31.1 to 51.4 nM and BLQ to 4.9 nM at hours 1, 3 and 6, respectively (fig 1B, table 1). Latanoprost acid was BLQ in both 12 h samples. Latanoprost acid levels were higher than bimatoprost acid levels at the 1, 3 and 6 h time points. The maximum mean level of latanoprost acid detected (41.3 nM at hour 3) was six times as high as the maximum mean level of bimatoprost acid detected (6.7 nM at hour 3).
Consistent with previous findings that latanoprost is rapidly and efficiently hydrolysed to its acid metabolite,6 none of the aqueous samples from patients treated with topical latanoprost contained quantifiable levels of intact latanoprost. By contrast, 1 and 3 h aqueous samples from patients treated with topical bimatoprost contained measurable levels of non‐metabolised drug (table 2). The mean concentration of bimatoprost was 6.6 and 2.4 nM in the 1 and 3 h samples, respectively, comparable to the concentration of bimatoprost acid (table 1).
Table 2 Intact drug levels in aqueous humour samples from patients with cataract.
| Intact drug levels* | ||||
|---|---|---|---|---|
| Hour 1 | Hour 3 | Hour 6 | Hour 12 | |
| Bimatoprost levels after bimatoprost treatment (n = 8 at each time point) | ||||
| Mean (SD) | 6.6 (3.6) nM | 2.4 (3.0) nM | 0 (0) nM | 0 (0) nM |
| Median | 7.6 nM | 1.5 nM | 0 nM | 0 nM |
| Range | BLQ–10.5 nM | BLQ–8.7 nM | All samples BLQ | all samples BLQ |
| Latanoprost levels after latanoprost treatment (n = 2 at each time point) | ||||
| Mean (SD) | 0 (0) nM | 0 (0) nM | 0 (0) nM | 0 (0) nM |
| Median | 0 nM | 0 nM | 0 nM | 0 nM |
| Range | Both samples BLQ | Both samples BLQ | Both samples BLQ | Both samples BLQ |
BLQ, below the limit of quantitation.
*Samples with intact drug BLQ (limit of quantitation was 0.5 ng/ml or 1.2 nM for bimatoprost and 1.0 ng/ml or 2.3 nM for latanoprost) were assigned a value of 0 nM.
Control assays confirmed the validity of the assay method. All samples were analysed for bimatoprost and bimatoprost acid levels, and no bimatoprost or bimatoprost acid could be quantified in samples from placebo‐treated or latanoprost‐treated patients. Quality control samples were included in all runs to ensure that assay performance met quality control acceptance criteria, and all showed accuracy within 88–112% of the nominal value.
Discussion
Our study found substantially higher levels of acid metabolite after a single dose of latanoprost than after a single dose of bimatoprost, although the concentration of drug in the bottle was six times higher with bimatoprost (0.03%) than with latanoprost (0.005%). Unlike latanoprost, which was completely hydrolysed, bimatoprost was present in aqueous samples at levels comparable to those of its acid metabolite.
These results are generally consistent with previous studies of acid metabolite levels in aqueous humour of patients with cataract who had been treated with bimatoprost or latanoprost.6,7 Camras et al7 evaluated aqueous samples from patients with cataract who were treated with once‐daily bimatoprost or vehicle for 1 week before surgery. The maximum mean level of bimatoprost acid measured was 22 nM, approximately threefold the maximum mean level (6.7 nM) measured in this study. Sjoquist and Stjernschantz6 evaluated aqueous samples from patients with cataract who were treated with latanoprost at 0.5–24 h before surgery. Latanoprost acid was detected by radioimmunoassay in all aqueous samples and reached a maximum level of about 100 nM,6 approximately 2.5‐fold the maximum mean level measured after a single dose of latanoprost in this study (41.3 nM).
Possible explanations for the differences in measured levels of acid metabolites across studies may include accumulation of acid metabolites during multiple dosing and different assay methods. Nonetheless, when the results of our study and the previous studies are examined together, acid metabolite levels are clearly higher after latanoprost treatment than after bimatoprost treatment.
Bimatoprost acid and latanoprost acid have shown similar functional potency at prostaglandin FP receptors in most normal (non‐transfected) human cells, with reported 50% effective concentration values of 26–112 nM for bimatoprost and 35–59 nM for latanoprost in fibroblasts and trabecular meshwork cells (table 3).
Table 3 Functional potency of acid metabolites at prostaglandin FP receptors in non‐transfected human cells.
| Effect measured | Cell type | EC50 (nM) | Reference | |
|---|---|---|---|---|
| Bimatoprost acid | Latanoprost acid | |||
| Ca2+ mobilisation | Human fibroblasts | 44 | 59 | (Allergan data on file) |
| Phosphoinositide hydrolysis | Human trabecular meshwork cells | 112 | 35 | Sharif et al8 |
| Phosphoinositide hydrolysis | Human trabecular meshwork cells | 26 | 35 | Sharif et al9 |
| Phosphoinositide hydrolysis | Human ciliary muscle cells | 4 | 198 | Sharif et al10 |
| Phosphoinositide hydrolysis | Human ciliary muscle cells | 4 | 124 | Sharif et al9 |
EC50, 50% effective concentration.
The exception seems to be human ciliary muscle cells. Bimatoprost acid was markedly more potent in these cells than in other cell types, and the activity of latanoprost acid was unexpectedly low.9,10 The 50% effective concentration of 124–198 nM for latanoprost acid in the ciliary muscle would suggest that a 100 nM aqueous humor concentration would be insufficient to decrease IOP. Further studies are needed because these findings are inconsistent with clinical findings of latanoprost acid levels and IOP lowering observed after latanoprost treatment, as well as with laboratory findings that 100 nM latanoprost acid is more effective than 100 nM bimatoprost acid in stimulating mitogen‐activated protein kinase in human ciliary muscle cells.10
Bimatoprost reduces IOP in humans through enhancement of pressure‐sensitive and pressure‐insensitive aqueous outflow.11,12 The molecular mechanism of action of bimatoprost in the trabecular meshwork is unknown. The maximal concentration of bimatoprost acid detected in aqueous humour samples in our study and in the previous study by Camras et al7 would not be sufficient for substantial activation of FP receptors on human trabecular meshwork cells. These findings suggest that bimatoprost hydrolysis and subsequent activation of FP receptors by bimatoprost acid is insufficient to account for the IOP‐lowering activity of bimatoprost.
In clinical studies, bimatoprost has consistently reduced IOP as well as or better than latanoprost,13,14,15 despite lower levels of the bimatoprost acid metabolite and the similar potency of bimatoprost acid and latanoprost acid at prostaglandin FP receptors.9,16 These clinical findings are consistent with the hypothesis that the ability of bimatoprost to lower IOP is independent of its hydrolysis to bimatoprost acid.
One limitation of this study is that drug levels were evaluated only in the aqueous humour. Data from in vitro human experiments suggest that topically applied bimatoprost reaches the ciliary body preferentially by scleral penetration, a route that is more direct than through the aqueous humour.17 In non‐human primates, the ciliary body is the target site of bimatoprost activity, and the effects of short‐term bimatoprost treatment on IOP can be explained entirely by an increase in uveoscleral outflow. Preclinical studies in primates using a single dose of topical drug at three times the clinical dosing concentration have shown that latanoprost is rapidly and efficiently converted to its free acid and achieves similar concentrations in the aqueous humour and ciliary body,18 whereas bimatoprost remains largely intact.2 Intact bimatoprost was present in the ciliary body at 10‐ to 100‐fold higher levels than in the aqueous humour, whereas bimatoprost acid levels were below the level of detection in the ciliary body. These results highlight the difference in drug metabolism and distribution profiles between bimatoprost and latanoprost, and confirm that in non‐human primates, non‐metabolised bimatoprost seems to be responsible for the IOP lowering observed with bimatoprost.
The question of whether bimatoprost is a prodrug like latanoprost in human eyes or has intrinsic activity has been the subject of debate. When we take into account the aqueous humour concentrations of bimatoprost acid and latanoprost acid (a lower concentration of bimatoprost acid), their similar potency at prostaglandin FP receptors, and the known IOP‐lowering efficacy of bimatoprost, we conclude that our results and the results of Camras et al7 are consistent with a hypothesis that bimatoprost is not a prodrug but, instead, works directly to reduce IOP. We acknowledge, however, that because a limited number of time points were used in our study, the true peak values of bimatoprost acid may be higher than were actually measured. Further, to fully address the role of bimatoprost hydrolysis in the mechanism of its IOP lowering, levels of bimatoprost and its acid metabolite must be measured in relevant target tissues. Future studies examining drug distribution and metabolism in the ciliary body are needed to understand the mechanism by which bimatoprost lowers IOP.
Abbreviations
BLQ - below the limit of quantitation
d4 - tetradeuterated
d5 - pentadeuterated
IOP - intraocular pressure
HPLC–MS/MS - high‐performance liquid chromatography–tandem mass spectrometry
PGF2α - prostaglandin F2α
Footnotes
Funding: This study was supported by unrestricted research grants from Allergan, and the Glaucoma Research and Education Foundation.
Competing interests: LBC has received research support from Allergan, Alcon Laboratories and Pfizer. He serves on the Speaker's Bureau and Glaucoma Advisory Board for Allergan. DW has received speaker honoraria from Alcon Laboratories and Pfizer. YC has received speaker honoraria from Alcon Laboratories and Pfizer. AA, DFW and LAW are employees of Allergan. JH, CWY, SV and AC report no conflicts of interest.
All authors contributed substantially to the conception and design of this study and had full access to the data. A medical writer, Kate Ivins, PhD, drafted the paper under the direction of the authors and was paid by Allergan. The paper was reviewed and approved by all authors. LBC and LAW act as guarantors and accept full responsibility for the integrity of the work.
All authors included in this paper fulfil the criteria of authorship, and there is no one else who fulfils the criteria but has not been included as an author.
References
- 1.Stjernschantz J W. From PGF(2alpha)‐isopropyl ester to latanoprost: a review of the development of Xalatan: the Proctor lecture. Invest Ophthalmol Vis Sci 2001421134–1145. [PubMed] [Google Scholar]
- 2.Woodward D F, Krauss A H, Chen J.et al Pharmacological characterization of a novel antiglaucoma agent, bimatoprost (AGN 192024). J Pharmacol Exp Ther 2003305772–785. [DOI] [PubMed] [Google Scholar]
- 3.Maxey K M, Johnson J L, LaBrecque J. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol 200247(Suppl 1)S34–S40. [DOI] [PubMed] [Google Scholar]
- 4.Davies S S, Ju W K, Neufeld A H.et al Hydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitro. J Ocul Pharmacol Ther 20031945–54. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker R F, Schoff E O, Nau C B.et al Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am J Ophthalmol 200113119–24. [DOI] [PubMed] [Google Scholar]
- 6.Sjoquist B, Stjernschantz J. Ocular and systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol 200247(Suppl 1)S6–12. [DOI] [PubMed] [Google Scholar]
- 7.Camras C B, Toris C B, Sjoquist B.et al Detection of the free acid of bimatoprost in aqueous humor samples from human eyes treated with bimatoprost before cataract surgery. Ophthalmology 20041112193–2198. [DOI] [PubMed] [Google Scholar]
- 8.Sharif N A, Kelly C R, Crider J Y. Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci 200344715–721. [DOI] [PubMed] [Google Scholar]
- 9.Sharif N A, Kelly C R, Crider J Y.et al Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther 200319501–515. [DOI] [PubMed] [Google Scholar]
- 10.Sharif N A, Crider J Y, Husain S.et al Human ciliary muscle cell responses to FP‐class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+ mobilization and MAP kinase activation. J Ocul Pharmacol Ther 200319437–455. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen G A, Nau C B, McLaren J W.et al Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in patients with ocular hypertension or glaucoma. Ophthalmology 20041111658–1662. [DOI] [PubMed] [Google Scholar]
- 12.Brubaker R F. Mechanism of action of bimatoprost (Lumigan). Surv Ophthalmol 200145(Suppl 4)S347–S351. [DOI] [PubMed] [Google Scholar]
- 13.Noecker R S, Dirks M S, Choplin N T.et al A six‐month randomized clinical trial comparing the intraocular pressure‐lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol 200313555–63. [DOI] [PubMed] [Google Scholar]
- 14.Parrish R, Palmberg P, Sheu W ‐ P.et al A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12‐week, randomized, masked‐evaluator, multicenter study. Am J Ophthalmol 2003135688–703. [DOI] [PubMed] [Google Scholar]
- 15.Simmons S T, Dirks M S, Noecker R J. Bimatoprost versus latanoprost in lowering intraocular pressure in glaucoma and ocular hypertension: results from parallel‐group comparison trials. Adv Ther 200421247–262. [DOI] [PubMed] [Google Scholar]
- 16.Sharif N A, Xu S X, Williams G W.et al Pharmacology of [3H]prostaglandin E1/[3H]prostaglandin E2 and [3H]prostaglandin F2alpha binding to EP3 and FP prostaglandin receptor binding sites in bovine corpus luteum: characterization and correlation with functional data. J Pharmacol Exp Ther 19982861094–1102. [PubMed] [Google Scholar]
- 17.Woodward D F, Krauss A H, Chen J.et al The pharmacology of bimatoprost (Lumigan). Surv Ophthalmol 200145(Suppl 4)S337–S345. [DOI] [PubMed] [Google Scholar]
- 18.Sjoquist B, Johansson A, Stjernschantz J. Pharmacokinetics of latanoprost in the cynomolgus monkey. 3rd communication: tissue distribution after topical administration on the eye studied by whole body autoradiography, Glaucoma research laboratories. Arzneimittelforschung 199949240–249. [DOI] [PubMed] [Google Scholar]