Abstract
Aim
To evaluate current clinical practice in the UK in the management of the anophthalmic socket; choice of enucleation, evisceration, type of orbital implant, wrap, motility pegging and complications.
Methods
All consultant ophthalmologists in the UK were surveyed by postal questionnaire. Questions included their practice subspecialty and number of enucleations and eviscerations performed in 2003. Specific questions addressed choice of implant, wrap, motility pegging and complications.
Results
456/896 (51%) consultants responded, of which 162 (35%) had a specific interest in oculoplastics, lacrimal, orbits or oncology. Only 243/456 (53%) did enucleations or eviscerations. 92% inserted an orbital implant after primary enucleation, 69% after non‐endophthalmitis evisceration, whereas only 43% did so after evisceration for endophthalmitis (50% as a delayed procedure). 55% used porous orbital implants (porous polyethylene, hydroxyapatite or alumina) as their first choice and 42% used acrylic. Most implants inserted were spherical, sized 18–20 mm in diameter. 57% wrapped the implant after enucleation, using salvaged autogenous sclera (20%), donor sclera (28%) and synthetic Vicryl or Mersilene mesh (42%). A minority (7%) placed motility pegs in selected cases, usually as a secondary procedure. 14% of respondents reported implant exposure for each type of procedure and extrusion was reported by 4% after enucleation and 3% after evisceration.
Conclusions
This survey highlights contemporary anophthalmic socket practice in the UK. Most surgeons use porous orbital implants with a synthetic wrap after enucleation and only few perform motility pegging.
Enucleation or evisceration is performed for various end‐stage eye diseases. The aim is to remove the diseased eye, provide adequate comfort, replace volume and give good functional and cosmetic appearance.
Two surveys have been conducted to evaluate trends in the management of the anophthalmic socket among oculoplastic surgeons in North America1,2 and one among ocularists in Germany.3 As there is sparse comparable information for the UK, we aimed to collect information of enucleation and evisceration management by postal survey of all consultant ophthalmologists in the UK regarding their preferred clinical practice.
Materials and methods
A questionnaire of anophthalmic socket management in the year 2003 was sent in April and May 2004 to all 896 consultant ophthalmologists in the UK, listed by The Royal College of Ophthalmologists, regardless of their subspecialty. Respondents were requested to return their completed questionnaire in a prepaid envelope, no reminders were sent and anonymity was maintained. Data management and analysis was performed using Microsoft Access and Excel software.
Results
Of the 896 consultant ophthalmologists in the UK, 456 responded giving a response rate of 51%. Of these 456, 321 (70%) had an interest in more than one ophthalmic subspecialty. But, overall, 135 (30%) had a special interest in one or more of oncology, oculoplastics, lacrimal or orbital surgery.
Enucleations or eviscerations were not performed in 2003 by 159 of 456 (35%). Referral to an oculoplastic surgeon was made by 125 (79%), with 70 of these within their own hospital and 4 within their own region. The subspecialty of the referring ophthalmologists included surgical retina (42 respondents), medical retina (37), glaucoma (26), paediatric ophthalmology (17), cornea or refractive (18), neuro‐ophthalmology (14) and other sub‐specialties (5).
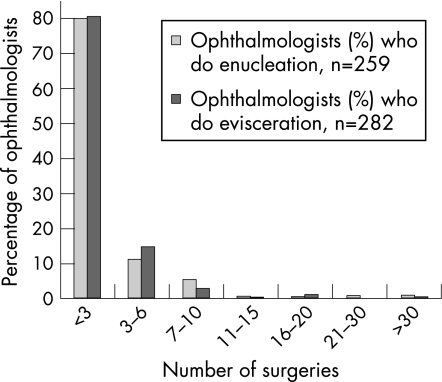
This type of surgery was performed by 65% (297/456) of respondents. Of these, 5% (16/297) did enucleations only, 13% (38/297) eviscerations only and 82% (243/297) did both. 80% (209/259) performed between one and three enucleations and eviscerations in the year, and 97% of enucleations and 98% of eviscerations were done by consultants performing <10 of each of these operations per year (fig 1). All consultants doing >7 enucleations or eviscerations per year (n = 21 consultants) had a special interest in one or more of oculoplastics, orbital, lacrimal or ocular oncology.
Figure 1 Summary of number of operations performed by surgeons in 2003. The majority have performed <3 surgeries.
Tables 1 and 2 calculate the total number of operations performed by the respondents. By using the median value for the number of operations performed and multiplying this value by the number of consultants in each group, a value for each type of surgery was obtained. This “minimum estimate” for enucleation was 718 cases and 699 for evisceration, for 2003 based on the responses from 51% of all the ophthalmologists in the UK.
Table 1 Results of number of enucleations performed by respondents in 2003, with a theoretical calculation for the minimum number of procedures in each group.
| Number of enucleations performed in 2003 | Median number of operations | Number of consultants | Derived theoretical total number of operations for each group |
|---|---|---|---|
| <3 | 1.5 | 209 | 313.5 |
| 3–6 | 4.5 | 29 | 130.5 |
| 7–10 | 8.5 | 14 | 119 |
| 11–15 | 13 | 2 | 26 |
| 16–20 | 18 | 1 | 18 |
| 21–30 | 25.5 | 2 | 51 |
| >30 | 30* | 2 | 60 |
| Total | 718 |
Calculation was derived by multiplying the median number of operations by each group by the number of consultants in each group.
*For the >30 group, it was not possible to use the median number, so 30 was used in the calculation.
Table 2 Results of number of eviscerations performed by respondents in 2003, with a theoretical calculation for the minimum number of procedures in each group.
| Number of eviscerations performed in 2003 | Median number of operations | Number of consultants | Derived theoretical total number of operations for each group |
|---|---|---|---|
| <3 | 1.5 | 227 | 340.5 |
| 3–6 | 4.5 | 41 | 184.5 |
| 7–10 | 8.5 | 9 | 76.5 |
| 11–15 | 13 | 1 | 13 |
| 16–20 | 18 | 3 | 54 |
| 21–30 | 25.5 | 0 | 0 |
| >30 | 30* | 1 | 30 |
| Total | 699 |
This was derived by multiplying the median number of operations by each group by the number of consultants in each group.
*For the >30 group, it was not possible to use the median number, so 30 was used in the calculation.
Ocular prosthetic services were available within their departments for 188 (41%) of the 456 respondents. A further 160 (35%) referred to National Artificial Eye service attending their own hospital or a nearby hospital, but 66 (14%) did not have prosthetic services in their unit and referred their patients elsewhere.
Specific surgical questions
Questions on the preferred surgical choice were completed by 297 respondents. Of these, 42% (125/297) preferred evisceration and 27% (79/297) preferred enucleation in any situation regardless of clinical scenario. The remaining 31% (93/297) selected evisceration or enucleation according to the clinical condition. When either enucleation or evisceration could be done, 70% (65/93) preferred to eviscerate and 13% (12/93) chose to enucleate; in painful blind eye secondary to end‐stage glaucoma 67% (62/93) preferred evisceration and 12% (11/93) enucleation; but in painful blind eye secondary to trauma 11% (10/93) preferred evisceration and 70% (65/93) enucleation.
Orbital implant insertion
After enucleation, 92% (239/259) of respondents inserted an orbital implant whereas only 43% (120/282) inserted an implant after evisceration for endophthalmitis and 69% (197/282) after evisceration for non‐endophthalmitis cases. Of the 120 consultants who insert an implant after evisceration for endophthalmitis, 61 (51%) preferred to do so as a primary procedure, 17 (14%) as a delayed procedure (within 6 weeks) and 38 (32%) as a secondary procedure after 6 weeks. Of the 30% (85/282) ophthalmologists who refer their patients for secondary implant after evisceration for non‐endophthalmitis cases, 12% (35/282) refer within their hospital and 6% (18/282) refer to other hospitals.
Type of implant
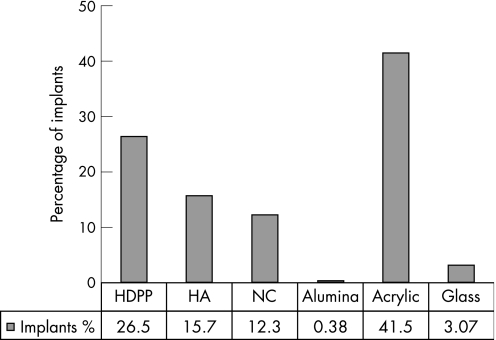
Of the 260 respondents, 183 (70%) used only one type of material for all cases, the most popular being acrylic (42%), but overall porous implant materials were of first choice in 56% of implants and 44% were non‐porous materials (fig 2). The second‐choice implant materials were acrylic for 11 ophthalmologists, glass for 2, natural coral for 8, porous polyethylene Medpor for 14 and synthetic hydroxyapatite for 7.
Figure 2 Summary of implants used after enucleations and evisceration. HA, hydroxyapatite; HDPP, high‐density porous polyethylene; NC, natural coral.
Most (89%) of the implants used were spherical in shape with the rest being hemispherical (9%) or conical (3%). Most (70%) preferred to use a 18–20 mm sized implant, 6% to use 16 mm implant, 3% to use 21 mm implant and 7% to use 22 mm implant. Only 10% used sizers to decide on the appropriate implant size and 4% preferred to use the biggest possible implant that fitted.
Type of wrap
Most implants (57%) were wrapped at placement in the orbit. Vicryl mesh (32%) is the most commonly used, followed by donor sclera (29%), salvaged sclera (22%), Mersilene mesh (13%) and others (5%) which include porcine collagen, fascia lata, bovine pericardium, prewrapped Vicryl, ocular muscles, Tenon's capsule or conjunctiva.
Muscle attachments
The majority (79%; 192/243) attached all four recti muscles to the implant and only 7% (17/243) attached all the six muscles to the implant, with the remainder attaching the four recti and the inferior oblique.
Pegging
The majority (93%, n = 215) of respondents do not use a motility peg. The low rate of pegging (7%, n = 17) was done mainly as a secondary procedure between 6 weeks and 2 years after implantation, with only four placing a motility peg as a primary procedure. However, none of the group who peg their implants do so in every case, only 3 do so for 31–50%, 2 for 11–20% and 11 for 1–10% of their cases. Complications from pegging occurred only with the consultants who peg 1–10% of their cases.
Complications
Overall, 14% of the respondents reported cases of exposure after either enucleation (22/161) or evisceration (18/128), 4% (7/161) reported extrusion after enucleation and 3% (4/128) after evisceration. Detailed analysis of these complications was limited in this survey due to questionnaire design, as specific clinical and surgical details were not requested. The materials used for secondary patch grafts to cover exposure were temporalis fascia, vicryl mesh, donor sclera, Mersilene mesh, orbital tissue, pericranium, buccal mucosa, hard palate and AlloDerm.
Discussion
We present the first survey of the management of anophthalmic sockets following enucleation and evisceration in the UK. The validity of this study is dependent on the accuracy of the responses we received and we have assumed that they were true accounts of contemporary surgical practice. Our response rate of 51% compares favourably with 23% and 31% for similar surveys in the USA in 1992 and 2003, respectively.1,2 This survey may not reflect the practice of all ophthalmologists in the UK and we do not claim to provide evidence of clinical superiority of any one particular procedure or technique over another.
Our survey reveals that 35% of respondents do not perform evisceration or enucleation and respondents who have an interest in oncology, oculoplastics, orbital or lacrimal surgery did >7 enucleations or eviscerations per year. This probably reflects the changing opinion among ophthalmologists that enucleation and evisceration should be done by subspecialist surgeons rather than by generalists.
This study shows that most of the ophthalmologists in the UK indicated that they preferred to eviscerate than enucleate in all situations, apart from painful eye following trauma. Paradoxically, however, from our survey (tables 1 and 2), we estimate roughly equal numbers of enucleations and eviscerations performed by our respondents, in contrast to the US experience of twice as many enucleations.2 Our estimate is a useful guide in that the numbers are not disparate and may demonstrate that UK practice differs to that in the US, though there is a chance that the few respondents performing >30 operations each year may skew the results if the true number is much greater.
Evisceration may be preferred for various reasons including better prosthetic motility and long‐term socket stability compared with enucleation.4,5,6 Only one case7 of sympathetic ophthalmia has been reported after evisceration in the last 25 years reflecting the rarity and improvement in treatment8,9 of sympathetic ophthalmia. On the other hand, enucleation is preferred as it provides histological diagnosis, more space for larger implants, better cosmesis, lower risk of extrusion and sympathetic ophthalmia.
Our survey found that the vast majority of respondents inserted an orbital implant after enucleation and after evisceration for non‐endophthalmitis cases. Insertion of an implant at the time of evisceration after endophthalmitis has recently been reported to be mostly successful,10 and meningitis following enucleation for endophthalmitis is virtually non‐existent in the modern era of systemic antibiotics.11
Regarding the choice of implant material, the majority of surgeons in the UK use porous spherical implants, similar to their US counterparts.2 Porous implants reduce the risk of infection, migration and extrusion.12 Porous implants have many advantages such as being light weight, better prosthetic retention and cosmesis and possible pegging. However, a porous implant is expensive when the final costs of anophthalmic socket management are totalled.13
Acrylic spheres were the single most commonly used type of implant. The cost‐effectiveness and similar prosthetic motility in comparison to unpegged porous implants14,15 accounts for the popularity of this material. Surgeons in Germany prefer alloplastic implant materials though their ocularists prefer to fit prosthesis where dermis fat graft has been used.3
Majority (57%) of our respondents preferred implant wrapping compared with 40% in the US survey.2 In the UK, Vicryl (polyglactin) mesh is the most popular wrap but donor and salvaged sclera are still used in small numbers. By contrast, in the US, donor sclera is the most popular material, used by 56% in 1992,1 but decreasing to 25% in 2003,2 followed by Vicryl (polyglactin) mesh (7%). The obvious advantage of synthetic materials is speed, but delayed vascularisation of sclera‐wrapped implants is believed to play a role in infection and exposure of the implant.16
Only a minority of respondents (7%) pegged porous implants, which is similar among oculoplastic surgeons in North America1,2 and Germany.3 Satisfactory prosthetic movement without pegging, additional surgery and complications17 due to pegging may influence the choice against pegging.
Some of the respondents reported cases of implant exposure and extrusion that are determined by many factors like infection, implant material and size, wrapping material, pegging, surgical technique used and surgeon's experience. Detailed analysis of complications was limited in this survey due to questionnaire design.
In conclusion, on the basis of the results of this survey, most surgeons in the UK indicated a preference for evisceration, though calculated figures indicate equality between evisceration and enucleation rates. After enucleation, the majority insert orbital implants. Porous implants are used more than non‐porous; synthetic wrapping materials are commonly used and only a minority of surgeons place motility pegs.
Footnotes
Competing interests: None.
This work was presented at the Annual Congress of The Royal College of Ophthalmologists, UK, May 2005 and European Society of Oculoplastic and Reconstructive Surgery Meeting, Crete, September 2005.
References
- 1.Hornblass A, Biesman B S, Eviatar J A. Current techniques of enucleation: a survey of 5,439 intraorbital implants and a review of literature. Ophthal Plast Reconstr Surg 19951177–86. [PubMed] [Google Scholar]
- 2.Su G W, Yen M T. Current trends in managing the anophthalmic socket after primary enucleation and evisceration. Ophthal Plast Reconstr Surg 200420274–280. [DOI] [PubMed] [Google Scholar]
- 3.Hintschich C, Baldeschi L. Rehabilitation of anophthalmic patients. Results of a survey. Ophthalmologe 20019874–80. [DOI] [PubMed] [Google Scholar]
- 4.Gurdal C, Erdener U, Irkec M.et al Incidence of sympathetic ophthalmia after penetrating eye injury and choice of treatment. Ocul Immunol Inflamm 200210223–227. [DOI] [PubMed] [Google Scholar]
- 5.Timothy N H, Freilich D E, Linberg J V. Evisceration versus enucleation from the ocularist's perspective. Ophthal Plast Reconstr Surg 200319417–420. [DOI] [PubMed] [Google Scholar]
- 6.Levine M R, Pou C R, Lash R H. The 1998 Wendell Hughes Lecture. Evisceration: is sympathetic ophthalmia a concern in the new millennium, Ophthal Plast Reconstr Surg 1999154–8. [DOI] [PubMed] [Google Scholar]
- 7.Griepentrog G J, Lucarelli M J, Albert D M.et al Sympathetic ophthalmia following evisceration: a rare case. Ophthal Plast Reconstr Surg 200521316–318. [DOI] [PubMed] [Google Scholar]
- 8.Kilmartin D J, Dick A D, Forrester J V. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol 200084259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan C C, Roberge R G, Whitcup S M.et al 32 cases of sympathetic ophthalmia. Arch Ophthalmol 1995113597–600. [DOI] [PubMed] [Google Scholar]
- 10.Dresner S C, Karesh J W. Primary implant placement with evisceration in patients with endophthalmitis. Ophthalmology 20001071661–1664. [DOI] [PubMed] [Google Scholar]
- 11.Afran S I, Budenz D L, Albert D M. Does enucleation in the presence of endophthalmitis increase the risk of postoperative meningitis? Ophthalmology 198794235–237. [DOI] [PubMed] [Google Scholar]
- 12.Moshfeghi D M, Moshfeghi A A, Finger P T. Enucleation. Surv Ophthalmol 200044277–330. [DOI] [PubMed] [Google Scholar]
- 13.Custer P L, Kennedy R H, Woog J J.et al Orbital implants in enucleation surgery: a report. Am Acad Ophthalmol 20031102054–2061. [DOI] [PubMed] [Google Scholar]
- 14.Custer P L, Trinkaus K M, Fornoff J. Comparative motility of hydroxyapatite and alloplastic enucleation implants. Ophthalmology 1999106513–516. [DOI] [PubMed] [Google Scholar]
- 15.Colen T P, Paridaens D A, Lemij H G.et al Comparison of artificial eye amplitudes with acrylic and hydroxyapatite spherical enucleation implants. Ophthalmology 20001071889–1894. [DOI] [PubMed] [Google Scholar]
- 16.Soparkar C N, Wong J F, Patrinely J R.et al Porous polyethylene implant fibrovascularization rate is affected by tissue wrapping, agarose coating, and insertion site. Ophthal Plast Reconstr Surg 200016330–336. [DOI] [PubMed] [Google Scholar]
- 17.Long J A, Tann TM I I I, Bearden WH I I I.et al Enucleation: is wrapping the implant necessary for optimal motility? Ophthal Plast Reconstr Surg 200319194–197. [DOI] [PubMed] [Google Scholar]