Abstract
Aim
To report the findings in a patient treated by repeated intravitreal bevacizumab (Avastin) injections, followed by macular relocation and excision of subfoveal choroidal neovascular membrane (CNV).
Methods
Histopathological evaluation of the CNV specimen, including immunohistochemical assessment.
Results
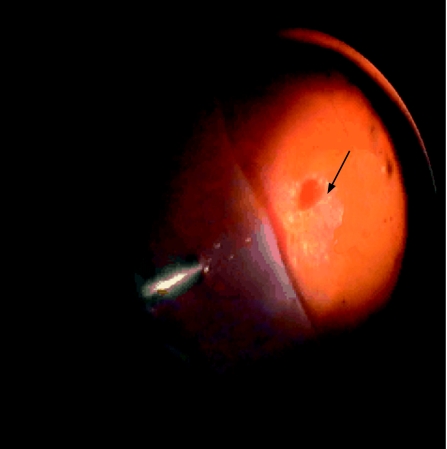
During surgical excision, the CNV seemed to be avascular and its underlying bed did not bleed. Histopathological examination revealed that the CNV comprised avascular fibrous subretinal tissue containing fibroblastic retinal pigment epithelial (RPE) cells, fragments of irregular thickened Bruch's membrane and fibrotic choroidal tissue containing some medium‐sized vessels but no choriocapillaris.
Conclusions
The development of an RPE tear during the course of Avastin treatment may reflect contraction of the avascular subretinal tissue, whereas the lack of capillaries in both choroidal and subretinal components may be caused by the increased access of Avastin to the choriocapillaris in the presence of the RPE tear.
In the era of increasing use of antivascular endothelial growth factor treatment in age‐related macular degeneration (AMD), we demonstrate histopathological correlations of clinical observations regarding the biological effects of bevacizumab on a choroidal neovascular membrane (CNV).
Patient and methods
A 74‐year‐old man underwent macular translocation surgery following repeated intravitreal bevacizumab (Avastin) injections. The first intervention consisted of pneumatic displacement of subretinal blood, followed by intravitreal bevacizumab injection in another unit. After a good initial visual response (20/40), the patient's vision dropped to 20/125, requiring a second injection. This was followed by a retinal pigment epithelial (RPE) tear. A third injection temporarily improved vision to 20/200, only for it to deteriorate again to 20/320 (26 letters at 2 m). Faced with the imminent loss of vision in his second eye, the patient chose the surgical option of macular translocation.
Macular translocation with 360° retinotomy was performed, consisting of phacovitrectomy, 360° retinotomy and 1300 centistokes silicone oil endotamponade, and excision of the CNV complex. This complex seemed to be avascular (fig 1) and the underlying bed hardly bled following excision. The excised tissue measured 4 mm in diameter. It was fixed in 10% neutral buffered formalin, dehydrated in ethanol and embedded in paraffin wax. Histopathological examination revealed that the CNV complex comprised three distinct components: fibrous subretinal tissue containing fibroblastic cells, fragments of irregular thickened Bruch's membrane and fibrotic choroidal tissue containing some medium‐sized vessels but no choriocapillaris (fig 2). Immunohistochemistry using cytokeratin 7 antibodies confirmed that the fibroblastic cells in the subretinal component were of RPE origin and that a few cells in the choroidal element were of a similar origin (fig 2). Immunohistochemistry for the endothelial marker CD34 confirmed the lack of vasculature in the subretinal part of the CNV (fig 2).
Figure 1 Relative avascularity of choroidal neovascular complex during surgical excision (black arrow). Retina folded nasally (left side).
Figure 2 (A) Periodic acid‐Schiff reagent and haematoxylin‐stained section of the excised tissue showing magenta‐coloured, irregular, thickened Bruch's membrane structure (arrows), discontinuous pigment cell layer (arrowheads), fibrous subretinal component (S) and fibrous choroidal remains (C). Inset: H&E staining of the specimen highlights its paucicellular nature. (B) Labelling with the retinal pigment epithelial (RPE) marker cytokeratin 7 demonstrates that the cells in the subretinal membrane are of RPE origin, as are a few in the choroidal scar (arrowheads). (C) Labelling with the vascular endothelial marker CD34 reveals that the subretinal component is avascular, whereas the choroid lacks capillaries, contains a few larger vessels (arrowheads) and is fibrotic. Inset: negative control section in which the primary antibody was replaced by an irrelevant antibody.
Eighty‐six days later, four‐muscle‐counter‐rotation surgery followed, including silicone oil removal. At 4 months following removal of silicone oil and muscle relocation surgery, the best‐corrected visual acuity was 20/80.
Discussion
We report the clinical and histopathological findings of a CNV excised during macular translocation after failure of intravitreal bevacizumab treatment.
Over‐expression of vascular endothelial growth factor (VEGF) in the RPE has been considered an important factor in the pathogenesis of choroidal neovascularisation in AMD.1 Bevacizumab is a humanised anti‐VEGF monoclonal antibody, which binds and inhibits all VEGF isoforms. This leads to a reduction in VEGF‐induced cell proliferation and tissue factor production.
Despite the lack of any phase III clinical trial data, there is an emerging practice of using bevacizumab for the treatment of choroidal neovascularisation. Short‐term studies suggest that this is a safe and effective treatment.2,3 However, our patient developed an RPE tear following the second bevacizumab injection. Although it has been stated that an RPE tear is not a frequent complication of the treatment,4 Meyer et al5 recently described two such cases following intravitreal bevacizumab injection.
A histopathological examination of the excised specimen following bevacizumab treatment indicated that it consisted of largely fibrotic choroid, irregular Bruch's membrane and an avascular subretinal RPE proliferation set in fibrous tissue. One of the most striking observations in the tissue was the lack of capillaries either in choroid or in the CNV elements. This finding correlated with the clinical observation that the CNV was avascular and with the lack of significant haemorrhage from the underlying bed after excision. However, as the RPE tear created a window defect with a resultant hyperfluorescence in the preoperative fundus fluorescein angiogram (fig 3), it was difficult to evaluate the status of the choriocapillaris before surgery.
Figure 3 Preoperative photographs. (A) Colour fundal photograph. (B) Early frames of fundal fluorescein angiogram depicting the window defect.
Absence of capillaries in CNV is not unusual, especially in the late stages of AMD.6 Equally, it is well recognised that the choriocapillaris may become partially atrophic in the same situation.7 However, a total absence of capillaries in both CNV and choroid is outside our previous experience of excised CNV complexes, even after photodynamic treatment.8 Thus, the combined histological and clinical findings raise the possibility that the avascularity reflects “withdrawal” of VEGF from the tissue after Avastin treatment. Indeed, it is well recognised that endothelial channels are highly susceptible to VEGF loss.9
The choroidal changes in our patient seemed to be restricted to the foveal area. If they were (partly) due to the Avastin treatment, the localisation to the fovea may be a consequence of the RPE tear. Such a tear may have permitted access of Avastin to the underlying choroid.
The histological appearances of the subretinal membrane are typical of a wound healing response involving RPE cells.10 From the clinical viewpoint, contraction associated with the wound healing response of the subretinal membrane may have contributed to the RPE tear. This possibility may be a concern for the use of Avastin in AMD presenting with a large fibrovascular PED.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascular membrane
RPE - retinal pigment epithelial
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None declared.
References
- 1.Lopez P F, Sippy B D, Lambert H M.et al Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age‐related macular degeneration‐related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 199637855–868. [PubMed] [Google Scholar]
- 2.Bashshur Z F, Bazarbachi A, Schakal A.et al Intravitreal bevacizumab for the management of choroidal neovascularization in age‐related macular degeneration. Am J Ophthalmol 2006142141–143. [DOI] [PubMed] [Google Scholar]
- 3.Rich R M, Rosenfeld P J, Puliafito C A.et al Short‐term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Retina 200626495–511. [DOI] [PubMed] [Google Scholar]
- 4.Fung A E, Rosenfeld P J, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 2006901344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer C H, Mennel S, Schmidt J C.et al Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) for occult choroidal neovascularisation secondary to age related macular degeneration. Br J Ophthalmol 2006901207–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossniklaus H E, Miskala P H, Green W R.et al Histopathologic and ultrastructural features of surgically excised subfoveal choroidal neovascular lesions: SST report no. 7. Arch Ophthalmol 2005123914–921. [DOI] [PubMed] [Google Scholar]
- 7.Garner A, Sarks S, Sarks J P. Degenerative and related disorders of the retina and choroid. In: Garner A, Klintworth GK, eds. Pathobiology of ocular disease: a dynamic approach. 2nd edn. New York: Marcel Dekker, 1994631–674.
- 8.Stanga P, Hiscott P, Li K.et al Macular relocation after photodynamic therapy for recurrent choroidal neovascular membrane: visual results and histopathological findings. Br J Ophthalmol 200387975–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo N, Mailhos C, Ju M.et al Inhibition of platelet‐derived growth factor B signaling enhances efficacy of anti‐vascular endothelial growth factor therapy in multiple models of ocular neovascularisation. Am J Pathol1682036–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent D, Sheridan C. Choroidal neovascularization: a wound healing perspective. Mol Vis9747–755. [PubMed] [Google Scholar]