Abstract
Background
Intraocular lens (IOL) implantation is becoming increasingly accepted as a primary procedure in infants.
Aim
To evaluate the accuracy of IOL power calculation, the rate of myopic shift and the refractive outcome after primary IOL implantation in infants aged <12 months at the time of cataract surgery.
Method
A retrospective case review of 25 patients (8 with bilateral cataracts and 17 with unilateral cataracts) who underwent cataract surgery with primary IOL implantation at <12 months of age. Outcomes measured were actual early postoperative refraction, lens power calculation error, myopic shift and refractive outcome.
Results
In 83% of cases, actual postoperative refraction was within 2 dioptres (D) of the target refraction. Lens power calculation error did not depend on axial length, age at surgery or target refraction. Mean (SD) myopic shift was 5.43 (3.7) D in the first 12 months after surgery, but was significantly greater when surgery was performed at <10 weeks of age.
Conclusion
This study demonstrates that IOL power can be calculated with reasonable accuracy in infants using current formulas. Factors such as age at the time of surgery, axial length, whether surgery is unilateral or bilateral, and the presence of systemic pathologies do not seem to influence the accuracy of lens power calculation or myopic shift up to 36 months of age.
Primary intraocular lens (IOL) implantation in infants is becoming increasingly acceptable owing to advances in surgical technique, improvements in IOLs and better understanding of the growth of the eye. Primary IOL implantation may allow optimal visual development by enhancing compliance with refractive correction. In infants with unilateral cataracts, primary IOL implantation reduces the anisometropic stimulus for amblyopia, and may therefore maximise the visual outcome, particularly where compliance with patching and refractive correction is poor.1 Primary IOL implantation in unilateral congenital cataracts has been previously shown to result in improved visual acuity, binocular vision and reduced strabismus compared with aphakia.2,3
Achieving a satisfactory long‐term refractive result after IOL implantation in infancy requires allowance for the rapid axial growth and myopic shift, which occurs during childhood.4,5,6,7 The rate of axial growth has been found to be slower in pseudophakic compared with aphakic eyes, yet the mean absolute quantity of myopic shift is greater in pseudophakic eyes because of the optical effect of the change in relative position of the IOL within the eye.8 The initial desired refractive outcome after IOL implantation is therefore hypermetropia, the amount of which depends on age of the child.9,10 An accurate refractive outcome after primary IOL implantation is crucial in order that large undesired refractive errors are avoided in later childhood and in adulthood. The common IOL power formulas Sanders–Retzlaff–Kraff (SRK)‐II, SRK‐T, Holladay and Hoffer‐Q have all been shown to give satisfactory refractive outcomes in paediatric cataract surgery, but are thought to be inaccurate in infants aged <36 months, and in those with axial lengths <20 mm.11,12
In this article, we present refractive data from primary IOL implantation in 33 eyes of 25 infants aged ⩽12 months at the time of cataract surgery.
Methods
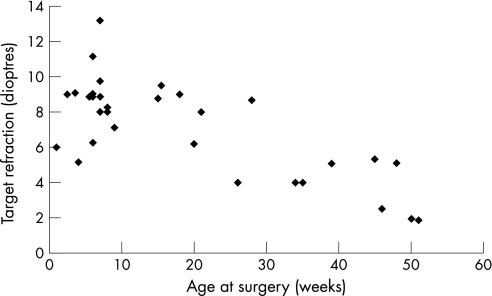
We undertook a retrospective case review of the notes of 25 patients (8 with bilateral cataracts and 17 with unilateral cataracts; 33 eyes) who underwent cataract surgery with primary IOL implantation. Biometry was performed under the same general anaesthetic as the cataract surgery. A‐scan (Quantel Medicus Axis II) was used to obtain axial length measurements, and a hand‐held keratometer was used to perform keratometry. The SRK‐T formula was used to calculate IOL power, in conjunction with lens manufacturers' A constants. The desired hypermetropic outcome was determined according to the patient's age at surgery (and refraction in the other eye where appropriate; fig 1).
Figure 1 Age at surgery and target refraction.
Statistical analysis
Statistical analysis was performed using SPSS for Windows statistical software V.11.5. Lens power calculation error was obtained by subtracting the target‐refractive outcome from the actual early postoperative value as measured at <4 weeks postoperatively (and usually at <2 weeks). Myopic shift at 12 months after surgery was calculated by subtracting the early postoperative refractive error from the refractive error at 12 months after surgery. Similarly, myopic shift at 36 months was calculated by subtracting the refractive error at 24 months after surgery from the refractive error at 36 months after surgery.
Results
Seventeen patients underwent unilateral cataract surgery (patients 1–17, table 1) and eight patients underwent bilateral cataract surgery (patients 18–25, table 1). The mean (SD) age at surgery was 18.09 (16.22) weeks (range 1–51 weeks corrected gestational age), and the mean (SD) axial length was 18.52 (1.8) mm (range 16–23 mm). The mean (SD) length of follow‐up was 44.36 (30.6) months (range 11–122 months). There were five patients with trisomy 21, four of whom had bilateral cataract surgery.
Table 1 Patient data.
| Patient number | Age at surgery (weeks) | Expected refraction (dioptres) | Actual refraction (dioptres) | Axial length (mm) | Coexistent pathology |
|---|---|---|---|---|---|
| 1 | 39 | 5.1 | 6 | 18.07 | Smith–Lemni–Opitz |
| 2 | 6 | 9.03 | 9.75 | 16.51 | |
| 3 | 7 | 8 | 8 | 18.09 | |
| 4 | 26 | 4 | 5 | 22.14 | Cutis marmorata, glaucoma |
| 5 | 28 | 8.68 | 10.5 | 18.12 | Foveal hypoplasia |
| 6 | 6 | 9 | 7 | 16.7 | Disc hypoplasia |
| 7 | 1 | 6 | 8.75 | 18.21 | |
| 8 | 18 | 9 | 8.5 | 18.44 | |
| 9 | 7 | 8.89 | 6.5 | 16.56 | |
| 10 | 7 | 13.2 | 14 | 15.9 | |
| 11 | 6 | 11.17 | 9.5 | 16.2 | |
| 12 | 7 | 9.76 | 7.5 | 16.56 | |
| 13 | 4 | 5.15 | 5.75 | 18.24 | Trisomy 21 |
| 14 | 6 | 6.27 | 5 | 19.44 | |
| 15 | 50 | 1.94 | 0.75 | ||
| 16 | 6 | 9.06 | 9.75 | 18.35 | |
| 17 | 8 | 8 | 7.5 | 18.99 | |
| 18 | 20 | 6.2 | 8 | 19.44 | |
| 21 | 8 | 10.25 | 19.46 | ||
| 19 | 34 | 4 | 4 | 21.16 | Cockaynes |
| 35 | 4 | 2.5 | 21.08 | Cockaynes | |
| 20 | 5.5 | 8.89 | 11 | 17.52 | Trisomy 21 |
| 6 | 8.89 | 10 | 18.13 | Trisomy 21 | |
| 21 | 15 | 8.77 | 8 | 18.5 | |
| 15.5 | 9.51 | 9 | 17.9 | ||
| 22 | 2.5 | 9 | 10 | 18.03 | Trisomy 21 |
| 3.5 | 9.1 | 11 | 18.05 | Trisomy 21 | |
| 23 | 48 | 5.1 | 4.5 | 18.25 | Galactosaemia |
| 45 | 5.33 | 4.25 | 18.24 | Galactosaemia | |
| 24 | 8 | 8.26 | 3.75 | 17.52 | Trisomy 21 |
| 9 | 7.11 | 5.25 | 17.08 | Trisomy 21 | |
| 25 | 46 | 2.5 | 1 | 22.7 | Trisomy 21 |
| 51 | 1.86 | 1 | 22.92 | Trisomy 21 |
Figure 1 shows the distribution of the target‐refractive outcomes that were aimed for according to the patients' age at the time of surgery. The mean (SD) target refraction was 7.23 (2.67) diopters (D) (range 1.86 to 13.20).
Lens power calculation error
The mean (SD) early postoperative refraction was 7.07 (3.23) D (range 0.75 to 14.00). The lens power calculation error ranged from +2.75 to −4.51 D, with a mean (SD) value of −0.167 (1.63) D. Expected and actual refractive errors were strongly correlated (p<0.001) and a paired t test showed no significant difference between the two variables (p = 0.559). In all, 45% of actual refractive errors fell within 1 D of the expected values and 81.8% of cases within 2 D.
We looked at factors that could predict whether our lens power calculation gave a satisfactory result, which we defined as being at a range of ±2 D from the target. Logistic regression analysis demonstrated how neither continuous (age, axial length and expected refraction; p = 0.068, 0.087 and 0.35, respectively) nor categorical predictors (unilateral or bilateral site of surgery, presence of systemic pathologies, association with trisomy 21; p = 0.63, 0.52, and 0.52, respectively) influenced the probability that actual refraction was at a range of ±2 D from the target (p = 0.352).
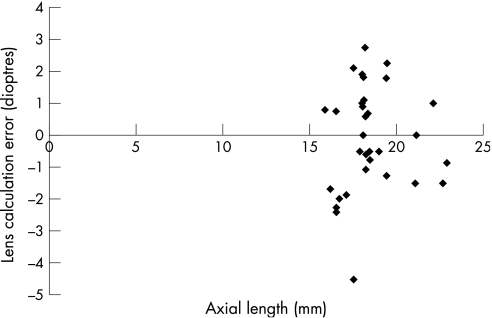
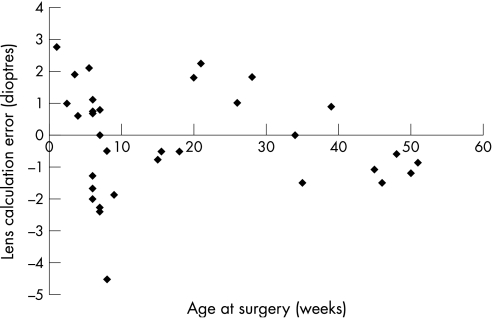
The mean (SD) lens calculation error was −0.533 (1.33) D for eyes with an axial length <20 mm and −0.572 (1.07) D for eyes >20 mm (p = 0.0526), which was not significantly different (fig 2). The mean (SD) lens calculation error in patients aged >26 weeks at the time of surgery was −0.445 (1.15) D and for those aged ⩽26 weeks old it was −0.617 (1.78) D, which was not significant (p = 0.713; fig 3). A total of 14 eyes were more hypermetropic than expected and 17 were more myopic.
Figure 2 Lens calculation error and axial length.
Figure 3 Lens calculation error and age at surgery.
Myopic shift
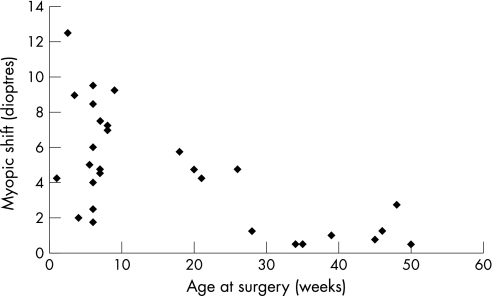
A paired t test demonstrated that the amount of myopic shift for all patients was greater in the first 12 months after surgery (5.43 (3.7) D) than in the third year (24–36 months postoperatively; 0.87 (0.62) D, p<0.001). An independent t test demonstrated that the amount of myopic shift in the first 12 months after surgery performed at <10 weeks of age (6.26 (2.91) D) was significantly greater than in the later surgery group (2.33 (1.99) D, p<0.001; fig 4). Similarly, the myopic shift in the first 12 months was significantly larger in patients with an axial length <20 mm (5.3 (3.05) D) than in larger eyes (1.75 (2.03) D, p = 0.033) and in patients with trisomy 21 (6.89 (3.77) D) than in other patients (4.11 (2.80) D, p = 0.045). There was no significant difference between unilateral and bilateral cases (p = 0.933) or between presence and absence of systemic pathologies as a whole (p = 0.414).
Figure 4 Myopic shift in first 12 months after surgery and age at surgery.
Thirty‐six months after surgery, there was no difference in the amount of myopic shift between the early and the late groups (p = 0.095), between the unilateral and the bilateral groups (p = 0.839), between the patients with and without systemic pathologies (p = 0.598) and, in particular, between the patients with trisomy 21 and other patients (p = 0.365).
Refractive outcome
Independent t tests showed that the refractive error at 18 months of age was significantly more myopic in patients with trisomy 21 (0.01 (3.85) D) than in those without (3.10 (3.60) DS, p = 0.05). However, by the age of 36 months, there was no significant difference in refractive error between patients with trisomy 21 and those without (p = 0.110).
χ2 Tests showed that there was no correlation between the amount of lens power calculation error and the amount of refractive error at 18 months (p = 0.243).
Discussion
This study shows that use of the lens power calculation formula SRK‐T gives satisfactory mean refractive outcomes in infants aged <12 months at the time of surgery, but with a wide range of errors. Other studies have also found a high degree of variability in lens calculation errors, particularly among the youngest patients with axial lengths <19 mm.12,13 This study demonstrates that the accuracy of lens power calculation is not influenced by age at the time of surgery, axial length, expected refraction, whether surgery is unilateral or bilateral, or the presence of systemic or other pathologies.
There is little published data on the rate of axial growth and refractive change in infants aged <12 months after primary IOL implantation. The mean quantity of myopic shift in aphakic eyes from age 3 months to 20 years has been shown to be 9.7 D.9 A mean myopic shift of 5.49 D in the first year has been shown to occur after IOL implantation in the first 12 months of life, by a study on 11 infants with unilateral congenital cataracts.14 Our results demonstrate a similar myopic shift of 5.43 (3.7) D in the first 12 months after surgery, which was significantly greater for patients who had surgery at <10 weeks of age. Other studies have demonstrated a greater myopic shift in unilateral pseudophakia in infants compared with bilateral cases,15 but we have found no significant difference between unilateral and bilateral cases.
Optimum visual development after cataract surgery with primary IOL implantation in infants requires appropriate refractive outcomes. Accuracy in biometry and knowledge of the factors affecting both the accuracy of biometry and the amount of myopic shift will help avoid high degrees of refractive error and anisometropia later in life and will improve visual outcomes for these patients. A greater understanding of the factors influencing lens power calculation errors is needed to avoid the high level of variability in error seen in infants.
Abbreviations
IOL - intraocular lens
SRK - Sanders–Retzlaff–Kraff
Footnotes
Competing interests: None declared.
References
- 1.Birch E E, Cheng C, Stager D R.et al Visual acuity development after the implantation of unilateral intraocular lenses in infants and young children. J AAPOS 20059527–532. [DOI] [PubMed] [Google Scholar]
- 2.Lambert S R, Lynn M, Drews‐Botsch C.et al Optotype acuity and re‐operation rate after unilateral cataract surgery during the first 6 months of life with or without IOL implantation. Br J Ophthalmol 2004881387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autrata R, Rehurek J, Vodickova K. Visual results after primary intraocular lens implantation or contact lens correction for aphakia in the first year of age. Ophthalmologica 200521972–79. [DOI] [PubMed] [Google Scholar]
- 4.Crouch E R, Crouch E R, Jr, Pressman S H. Prospective analysis of pediatric pseudophakia: myopic shift and postoperative outcomes. J AAPOS 20026277–282. [DOI] [PubMed] [Google Scholar]
- 5.Plager D A, Kipfer H, Sprunger D T.et al Refractive change in pediatric pseudophakia: 6‐year follow‐up. J Cataract Refract Surg 200228810–815. [DOI] [PubMed] [Google Scholar]
- 6.Enyedi L B, Peterseim M W, Freedman S F.et al Refractive changes after pediatric intraocular lens implantation. Am J Ophthalmol 1998126772–781. [DOI] [PubMed] [Google Scholar]
- 7.Vasavada A R, Raj S M, Nihalani B. Rate of axial growth after congenital cataract surgery. Am J Ophthalmol 2004138915–924. [DOI] [PubMed] [Google Scholar]
- 8.McClatchey S K, Dahan E, Maselli E.et al A comparison of the rate of refractive growth in pediatric aphakic and pseudophakic eyes. Ophthalmology 2000107118–122. [DOI] [PubMed] [Google Scholar]
- 9.McClatchey S K, Parks M M. Theoretic refractive changes after lens implantation in childhood. Ophthalmology 19971041744–1751. [DOI] [PubMed] [Google Scholar]
- 10.Dahan E. Intraocular lens implantation in children. Curr Opin Ophthalmol 20001151–55. [DOI] [PubMed] [Google Scholar]
- 11.Andreo L K, Wilson M E, Saunders R A. Predictive value of regression and theoretical IOL formulas in pediatric intraocular lens implantation. J Pediatr Ophthalmol Strabismus 199734240–243. [DOI] [PubMed] [Google Scholar]
- 12.Tromans C, Haigh P M, Biswas S.et al Accuracy of intraocular lens power calculation in paediatric cataract surgery. Br J Ophthalmol 200185939–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neely D E, Plager D A, Borger S M.et al Accuracy of intraocular lens calculations in infants and children undergoing cataract surgery. J AAPOS 20059160–165. [DOI] [PubMed] [Google Scholar]
- 14.Lambert S R, Buckley E G, Plager D A.et al Unilateral intraocular lens implantation during the first six months of life. J AAPOS 19993344–349. [DOI] [PubMed] [Google Scholar]
- 15.Gouws P, Hussin H M, Markham R. Long term results of primary posterior chamber intraocular lens implantation for congenital cataract in the first year of life. Br J Ophthalmol 200690975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]