Abstract
Aim
To detail the clinical findings in a British family with molecularly characterised Wagner syndrome.
Background
Only in the last year has the specific genetic defect in Wagner syndrome been identified, and the background literature of the molecular genetics is outlined. Clinical and laboratory findings in a second case of Wagner syndrome are included to highlight difficulties that can be encountered when identifying pathogenic mutations for disorders arising in complex genes.
Methods
Mutation screening was performed using PCR and RT‐PCR.
Results
A heterozygous mutation was found converting the donor splice site of exon 8 of the chondroitin sulphate proteoglycan 2 (CSPG2). This is the same mutation that has been reported in the original Wagner pedigree. The main clinical features of Wagner syndrome are vitreous syneresis, thickening and incomplete separation of the posterior hyaloid membrane, chorioretinal changes accompanied by subnormal electroretinographic responses, an ectopic fovea and early‐onset cataract. A clinical feature present in this family, but previously undescribed, is anterior uveitis without formation of synechiae. Wagner syndrome has a progressive course, resulting in loss of vision even in the absence of retinal detachment.
Conclusion
On a background of considerable confusion regarding the distinction between Wagner syndrome and predominantly ocular Stickler syndrome, it is now apparent the that two conditions are both clinically and genetically distinct. This report summarises the clinical findings in Wagner syndrome and extends the phenotypic characteristics.
Wagner vitreoretinal degeneration (Online Mendelian Inheritance in Man (OMIM) #143200) is one of the hereditary vitreoretinopathies. Historically there has been considerable debate on the classification of the hereditary vitreoretinopathies, in particular between Wagner and Stickler syndrome (OMIM #108300 #604841 #184840), and the diagnosis of vitreous diseases is often ambiguous.1,2,3 Molecular genetics has played an increasing role in identifying the underlying aetiology of the vitreoretinopathy syndromes, and the recent publication of a pathological mutation in the original Wagner pedigree confirmed the role of the chondroitin sulphate proteoglycan 2 (CSPG2) gene in Wagner syndrome.4 The aim of the present paper is to clarify the clinical findings in Wagner syndrome.
In 1938, Wagner described a vitreoretinal disorder with an autosomal dominant pattern of inheritance in a large Swiss pedigree.5 The syndrome has been described subsequently in other reports.6,7,8 In the original report, Wagner syndrome is characterised by vitreous syneresis, posterior strands and veils in the vitreous cavity, avascular membranes, and chorioretinal atrophy. It is also associated with early onset cataract and mild myopia. The chorioretinal pathology results in gradual progressive visual loss in the absence of retinal detachment, and has a progressive course.9
Wagner syndrome was first shown to be linked to chromosome 5q13–14 by Brown et al10 in 1995. Miyamoto et al11 found a mutation in intron 7 affecting the acceptor splice site of the alternatively spliced exon 8 of the CSPG2 gene that resulted in mis‐splicing and concluded that this was the Wagner locus.11 Subsequently, Kloeckener‐Gruissem et al4 identified a mutation in intron 8 of the original Wagner pedigree, and three further novel intron 7 sequence mutants in the CSPG2 gene have been reported in six Dutch families.12 All these are thought to affect correct splicing of exon 8. CSPG2 encodes the core protein of a proteoglycan called versican, which is expressed as several alternatively spliced isoforms.13 At least four major isoforms exist (V0, V1, V2 and V4) that vary in their inclusion or exclusion of exons 7 and 8 of the CSPG2 gene.13
Here we detail the clinical findings, with long‐term follow‐up, in a father and daughter with Wagner syndrome and describe the molecular analysis that led to identification of the same mutation as the original Wagner pedigree. The same molecular analysis approach has failed to identify a genetic defect in a further, unrelated, case with clinically identical features. The clinical and laboratory findings are detailed for this second case to highlight the difficulties that can be encountered in the identification of pathogenic mutations for disorders arising in complex genes with multiple spliced isoforms.
Materials and methods
Clinical examination
Patient A, patient A's daughter and patient B were under regular follow‐up in the ophthalmic department of Addenbrookes hospital, Cambridge, UK. Patient A presented in 1977. Patient B had been under follow‐up for 20 years. Informed consent was obtained for photographs and molecular genetic analysis.
Molecular analysis
Cultured skin fibroblasts incubated either with or without emetine to inhibit non‐sense‐mediated decay14 were used to prepare RNA that was reverse transcribed with superscript II (Invitrogen, Paisley, UK) and amplified with TaqPlus (Stratagene, La Jolla, CA, USA) using primers derived from the reference sequences NM_004385 (V0 full‐length transcript). Genomic DNA prepared from blood leucocytes was amplified using primers derived from the reference sequence NT_086677. Exons 7 and 8 were amplified individually with AccuTaq (Sigma‐Aldrich, St Louis, MO, USA). These cDNA and genomic DNA products were used to sequence the complete open reading frame and the splice sites adjacent to exons 7 and 8. In addition, for patient B, genomic DNA was used to amplify and sequence all 15 exons and their surrounding splice site sequences. Big Dye Terminator V.3.1 (Applied Biosystems, Foster City, California, USA) was used in the sequencing reactions and then analysed on a 3130xl sequencing machine (Applied Biosystems,).
Controls consisting of 46 unrelated individuals (92 chromosomes) were analysed for a mutation found in family A. An amplified genomic product of around 350 bp containing the mutation site was incubated with the enzyme SexA I at 37°C for 16 h and then analysed by electrophoresis in a Nusieve agarose gel, followed by staining with ethidium bromide and visualisation under ultraviolet (UV) light. The enzyme cut the normal sequence but did not cut the mutant allele.
To attempt to identify if cryptic splice sites were being utilised instead of the mutated donor splice site in patient A, RT‐PCR (as described previously) was used to amplify complementary DNA (cDNA) using primers within exon 8. A sense primer was used to amplify from the 5' end of exon 8 to exon 9, while an antisense primer was used to amplify from the 3' end of exon 8 to exon 7. These amplified cDNAs were analysed by sequencing.
Results
Clinical history—patient A (male) and daughter
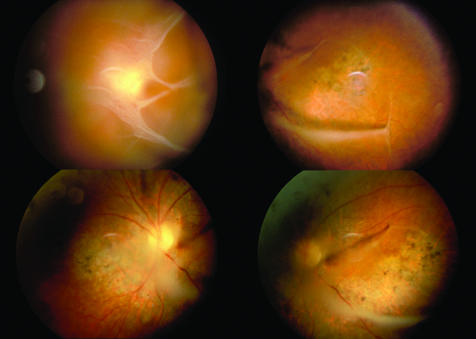
Patient A, aged 37 years, presented with a 3‐month history of monocular diplopia in the left eye, with visual acuities 6/6R and 6/9L. There was a strong family history of cataract on his father's side. Fundus examination on presentation showed curious bilateral vitreous membranes and scattered pigmentation in both lower fundi (fig 1). One year later the left nuclear cataract and right chorioretinal atrophy had worsened with increased retinal pigmentation and some pre‐retinal haemorrhage. Flare and cells were noted in the anterior chamber of the left eye, but the lens opacity remained nuclear, with no posterior subcapsular element. At 2 years, visual acuities had deteriorated to 6/12R and 6/18L. The uveitis persisted without synechiae. A left extracapsular cataract extraction was performed, and best corrected visual acuity (with contact lens +11.00 D) post‐cataract extraction was 6/12. Six years later (aged 46 years), right cataract surgery with posterior lens implant was performed. Posterior capsule opacification was treated with YAG laser capsulotomy, but the visual acuity did not improve beyond 6/60 and deteriorated gradually over subsequent years to no light perception in 2001. Visual acuity also gradually deteriorated in the left eye to 6/60 with contact lens. A flash electroretinogram (ERG) at age 55 years showed gross abnormality of both rod and cone responses in both eyes, but more marked on the right than on the left (fig 2). Raised intraocular pressure following the cataract extractions has become increasingly difficult to control and three drainage operations have been performed in the right eye.
Figure 1 Fundal images of patient A showing the vitreous condensations and chorioretinal atrophy characteristically described in Wagner syndrome.
Figure 2 Rod and cone electroretinograms. (A) Representative normal responses; (B) responses from the left eye of patient A, aged 55 years; and (C) responses from the left eye of patient A's daughter, aged 19 years. The figure therefore shows the traces from the better eye of both patients following uncomplicated cataract extraction (B) and before any surgery (C). The top two traces are light‐adapted cone responses to 30 Hz flicker and to a bright flash; the lower four traces are dark‐adapted rod and mixed rod‐cone responses to a range of stimuli intensities from dim (bottom) to bright (4th from bottom).
Patient A's daughter presented with night blindness and was found to have a large pseudosquint (fig 3). Best corrected visual acuity at age 12 years was 6/12 bilaterally. The same striking fundal findings of bilateral vitreous membranes and chorioretinal atrophy as seen in her father were found on fundal examination. Flash ERG performed at age 19 years revealed markedly subnormal rod and cone responses bilaterally, consistent with an extensive retinal abnormality affecting photoreceptor layers (fig 2). Bilateral anterior uveitis without synechiae has also been a feature in the daughter's clinical history requiring intermittent topical treatment. Uncomplicated phakoemulsification cataract extractions with posterior lens implants were performed at age 26 years. At surgery, the anterior capsules were found to appear highly elastic, and subsequent recurrent anterior and posterior capsulofibrosis has required multiple YAG laser capsulotomies. In both father and daughter, the retinas have remained flat, with no breaks and no retinal detachments.

Figure 3 Image of patient A's daughter showing the pseudosquint measured as a large positive angle kappa. Patient consent was obtained for publication of this photograph.
Clnical history—patient B
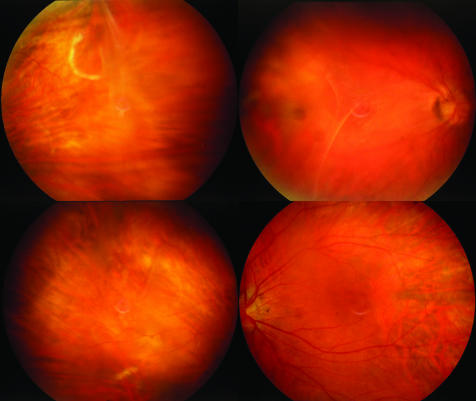
Patient B had been myopic since the age of 2 years. There was no family history of visual problems on her father's side, but her mother, who is now deceased, had been blind. Patient B also exhibited a pseudodivergent squint with a large angle kappa. In her late 30s, she developed poor night vision and sluggish adaptation moving from differing lighting levels. At 38 years of age she had a retinal detachment in her left eye, and required further surgery for left retinal reattachment at age 40 years. Cataract surgery was performed on the right eye for accelerated nuclear sclerotic cataract at age 50 years. This improved the visual acuity from 6/24 (with −9.50/−1.50×15) to 6/6 (with −0.75/−1.00×10), with a 13.5 D lens. The left vision was reduced to perception of light. Fundoscopy of the right eye, which has had no retinal surgery, and where the posterior hyaloid membrane has incompletely separated, has shown it to be particularly thickened (fig 4), with areas of erosive chorioretinopathy that are similar to, but less pronounced than, those seen in patient A.
Figure 4 Fundal images of patient B.
DNA analysis
In both patients A and B, amplification of cDNA preferentially resulted in the smallest splice isoform that did not include sequence from either exon 7 or 8. Sequencing of this cDNA resulted in only the normal sequence or previously documented polymorphisms.
Exons 7 and 8 including the splice sites were amplified from genomic DNA for both patients A and B. Sequences for these were normal except for the donor splice site of intron 8 in patient A. Here, a heterozygous mutation was found converting the dinucleotide gt→at (X15998:‐c0.6570+1g→a). The mutation was also found in patient A's affected daughter, but was absent in 92 control chromosomes. Kloeckener‐Gruissem et al4 have also reported 100 controls in whom this mutation was not present. Although the V3 isoform is preferentially expressed in the cultured fibroblasts, it is possible to specifically amplify cDNAs containing exon 8 by using primers located within it. This approach was used to search for the use of cryptic splice sites as documented by Miyamoto et al11 and Kloeckener‐Gruissem et al.4 Amplified cDNA products containing exon 8 extending either upstream to exon 7 or downstream to exon 9 were amplified and sequenced, but we did not detect mis‐splicing caused by utilisation of cryptic splice sites, particularly the 21 bp deletion noted by Kloeckener‐Gruissem et al.4
As we did not identify a mutation in patient B with this initial approach, the genomic sequencing was extended to include 500 base pairs upstream and downstream of exon 7 and exon 8 and the remaining 13 exons, including their splice sites. Only previously documented polymorphisms were identified. Although we have seen in our experience that mutations in collagen, type II, alpha 1 (COL2A1) are usually associated with a type 1 retrolental vitreous anomaly (which was not present in this case), in light of one previous report of Wagner‐like clinical findings with a COL2A1 mutation in exon 2,15 screening of this COL2A1 exon was undertaken in patient B, but this was also normal.
Discussion
Versican is a chondroitin sulphate proteoglycan expressed in many tissues including the vitreous gel, where it represents 5–15% of the total protein content.16 The amino‐terminal of versican binds to hyaluronan, resulting in the formation of very large complexes, and the carboxy‐terminal end consists of two epidermal growth factor domains with a complement regulatory region.17 Hyaluronan‐binding proteoglycans such as versican have been shown to play an important role in maintaining the structural integrity of tissues. In addition, chondroitin sulphate proteoglycans have been implicated in cell adhesion, cell migration, cell proliferation, and regulation of cellular activities.13
Determining the exact function of versican in various tissues is complicated as the proteoglycan has at least four major splice variants; V0 contains two glycosaminoglycan attachment regions located centrally on the molecule and encoded by exons 7 and 8. V1 utilises exon 8 but not 7, V2 contains exon 7 but not 8, and V3 uses neither exon 7 nor 8 and so has no potential glycosaminoglycan attachment sites.
For correct splicing to occur, the three main splicing control elements of the acceptor, donor and branch sites of an exon are required, along with a number of other, less well‐understood elements which can be located either in the intron or in the exon. There are, therefore, a number of mechanisms by which a mutation can affect splicing, and apparently silent mutations may be having a pathogenic effect through these other elements that are necessary for correct messenger RNA (mRNA) processing.18 Miyamoto et al11 demonstrated some utilisation of a cryptic acceptor splice site within exon 8 of the mutant allele, but their data also suggested complete exon skipping. Because of the preferential amplification of the much smaller V3 splice isoform, it is difficult to directly demonstrate exon 8 skipping. We were unable to identify utilisation of a cryptic splice site within exon 8 in patient A, and this may reflect the different cells analysed by us (cultured skin fibroblasts compared with cultured lymphoblastoid cells or whole blood RNA used by others4,11). However, Mukhopadhyay et al12 found a significant increase of CSPG2/versican V2 and V3 isoforms, indicating skipping of exon 8 which is present in the V0 and V1 transcripts,12 and our results suggest the same. Mukhopadhyay et al proposed a pathogenic mechanism in Wagner syndrome based on a reduction in the number of chondroitin sulphate side chains of versican.12
Clinically, the most striking finding in Wagner syndrome is thickening and incomplete separation of the posterior hyaloid membrane, which tends to occur in a circular band and is variously described as veils, sheets or ropes.9,10 A large range of chorioretinal abnormalities have been described in Wagner syndrome, with the typical finding being chorioretinal atrophy with pigment migration into the retina. Electroretinographic responses are progressively subnormal, and visual field testing demonstrates ring scotomas with eventual loss of central visual acuity in a picture similar to retinitis pigmentosa.6 Both patient A's daughter and patient B detailed in the present paper reported nyctalopia. The electrophysiologically confirmed retinal dysfunction in Wagner syndrome is in contrast with findings in the most common hereditary vitreoretinopathy Stickler syndrome. The retinal changes in Stickler syndrome consist of retinal tears (with a particular predisposition to giant retinal tears) leading to rhegmatogenous retinal detachment and paravascular pigmented degeneration. Retinal function in Stickler syndrome is normal and visual loss is not progressive, unlike Wagner syndrome.
In the original Wagner pedigree, 54% of 56 affected eyes had an abnormal “dragged” appearance to the central retinal vessels. Miyamoto et al11 postulated that pseudostrabismus due to an ectopic fovea may be a previously unrecognised manifestation of Wagner syndrome. Other reports do not comment on this, but at least two individuals in the original pedigree re‐examined in the early 1980s are reported to have presented with a large positive angle kappa.19 Both patient A's daughter and patient B, characterised here also had a large positive angle kappa, which would support an ectopic fovea as a further feature associated with Wagner syndrome. It is not known why patient A developed glaucoma. Raised intraocular pressure is not a reported association with Wagner syndrome and, in patient A, may not be a consequence of the mutation in CSPG2. Drainage angle anomalies have been reported in other inherited vitreoretinopathies like Stickler syndrome, but, in our experience, this is uncommon and the incidence of glaucoma in Wagner syndrome is unknown.
A characteristic of the history in patient A and his daughter is longstanding anterior uveitis without synechiae. Aggressive lens capsule opacification following uncomplicated cataract surgery with posterior lens implant was noted in both clinical histories. The onset of visual loss varies in Wagner syndrome as demonstrated in patient A and patient B (late visual loss at age 50 years) in contrast with early visual loss in the daughter of patient A (at age 20 years).
Erosive vitreoretinopathy (ERVR; OMIM 143200) was reported as a new clinical entity in 1994.20 The five‐generation family described showed an autosomal dominant pattern of inheritance. The vitreous findings were marked syneresis with prominant membranes. Nyctalopia was accompanied by retinal pigment epithelial change and grossly reduced rod and cone ERG responses, and progressive chorioretinal atrophic changes were also described. A feature in this family was combined traction‐rhegmatogenous retinal detachments occurring in 11 of 15 affected individuals. The presence of large angle kappas in affected family members is worth noting.20 The clinical findings appear very similar to those of Wagner syndrome, and the time course of visual loss in the affected family members with erosive vitreoretinopathy is consistent with both that described in the original Wagner family and our experience. Furthermore, linkage studies have subsequently found that the region segregating to erosive vitreoretinopathy overlaps the critical region found for Wagner disease.10 As commented by Mukhopadhyay et al,12 it now seems likely that Wagner syndrome and erosive vitreoretinopathy are allelic and are merely phenotypic variants of the same disorder.
By describing two members of a family and an unrelated individual, we have attempted to highlight the clinical features and potential difficulties of genetic analysis of Wagner syndrome. Despite a strong clinical diagnosis of Wagner syndrome in patient B, no pathological mutation was identified. With the difficulty in determining splicing defects in large complex genes that produce multiple splice variants, it may not be possible to easily identify a pathological mutation in every patient presenting with the disorder, or, like Stickler syndrome, Wagner vitreoretinal degeneration may yet prove to be genetically heterogeneous.
Acknowledgements
We thank Keith Bradshaw for the electrophysiology data, and Gill Whitmore for administrative help.
Abbreviations
cDNA - complementary DNA
COL2A1 - collagen, type II, alpha 1
CSPG2 - chondroitin sulphate proteoglycan 2
ERG - electroretinogram
OMIM - Online Mendelian Inheritance in Man
Footnotes
Funding: Action Medical Research (ref S/P/3937), The Evelyn Trust.
Competing interests: None.
References
- 1.Billington B M, Leaver P K, McLeod D. Management of retinal detachment in the Wagner‐Stickler syndrome. Trans Ophthalmol Soc UK 1985104875–879. [PubMed] [Google Scholar]
- 2.Zlotogora J, Sagi M, Schuper A.et al Variability of Stickler syndrome. Am J Ophthalmol 199242337–339. [DOI] [PubMed] [Google Scholar]
- 3.Korkko J, Ritvaniemi P, Haataja L.et al Mutation in type II procollagen (COL2A1) that substitutes aspartate for glycine alpha1‐67 and that causes cataracts and retinal detachment: evidence for molecular heterogeneity in the Wagner syndrome and the Stickler syndrome (arthro‐ophthalmopathy). Am J Hum Genet 19935355–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Kloeckener‐Gruissem B, Bartholdi D, Abdou M ‐ T.et al Identification of the genetic defect in the original Wagner syndrome family. Mol Vis 200612350–355. [PubMed] [Google Scholar]
- 5.Wagner H. Ein bisher unbekanntes Erbleiden des Auges (Degeneratio hyaloideo‐retinalis hereditaria), beobachtet im Kanton Zurich. Klin Monatsbl Augenheilkd 1938100840–857. [Google Scholar]
- 6.Fryer A E, Upadhyaya M, Littler M.et al Exclusion of COL2A1 as a candidate gene in a family with Wagner‐Stickler syndrome. J Med Genet 19902791–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zech J C, Morle L, Vincent P.et al Wagner vitreoretinal degeneration with genetic linkage refinement on chromosome 5q13–q14. Graefe's Arch Clin Exp Ophthalmol 1999237387–393. [DOI] [PubMed] [Google Scholar]
- 8.Perveen R, Hart‐Holden N, Dixon M J.et al Refined genetic and physical localization of the Wagner disease (WGN1) locus and the genes CRTL1 and CSPG2 to a 2‐ to 2.5‐cM region of chromosome 5q14.3. Genomics 199957219–226. [DOI] [PubMed] [Google Scholar]
- 9.Graemiger R A, Niemeyer G G, Schneeberger S A.et al Wagner vitreoretinal degeneration. Follow‐up of the original pedigree. Ophthalmology 19951021830–1839. [DOI] [PubMed] [Google Scholar]
- 10.Brown M B, Graemiger R A, Hergersberg M.et al Genetic linkage of Wagner disease and erosive vitreoretinopathy to chromosome 5q13–14. Arch Ophthalmol 1995113671–675. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto T, Inoue H, Sakamoto Y.et al Identification of a novel splice site mutation of the CSPG2 gene in a Japanese family with Wagner syndrome. Invest Ophthalmol Vis Sci 2005462726–2735. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay A, Nikopoulos K, Maugeri A.et al Erosive vitreoretinopathy and Wagner disease are caused by intronic mutations in CSPG2/versican that result in an imbalance of splice variants. IOVS 2006473565–3572. [DOI] [PubMed] [Google Scholar]
- 13.Wight T N. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 200213617–623. [DOI] [PubMed] [Google Scholar]
- 14.Carter M S, Doskow J, Morris P.et al A regulatory mechanism that detects premature nonsense codons in T‐cell receptor transcripts in vivo is reversed by protein synthesis inhibitors in vitro. J Biol Chem 199527028995–29003. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S K, Leonard B C, Damji K F.et al A frame shift mutation in a tissue‐specific alternatively spliced exon of collagen 2A1 in Wagner's vitreoretinal degeneration. Am J Ophthalmol 2002133203–371. [DOI] [PubMed] [Google Scholar]
- 16.Theocharis A D, Papageorgakopoulou N, Feretis E.et al Occurrence and structural characterisation of versican‐like proteoglycan in human vitreous. Biochimie 2002841235–1241. [DOI] [PubMed] [Google Scholar]
- 17.LeBaron R G, Zimmermann D R, Ruoshlahti E. Hyaluronate binding properties of versican. J Biolog Chem 199226710003–10010. [PubMed] [Google Scholar]
- 18.Ladd A N, Cooper T A. Finding signals that regulate alternative splicing in the post‐genomic era. Genome Biol 200231–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maumee I H, Stoll H U, Mets M B. The Wagner syndrome versus hereditary ophthalmopathy. Trans Am Ophthalmol Soc 198280349–365. [PMC free article] [PubMed] [Google Scholar]
- 20.Brown D M, Kimura A E, Weingeist T A.et al Erosive vitreoretinopathy. A new clinical entity. Ophthalmology 1994101694–704. [DOI] [PubMed] [Google Scholar]