Abstract
Aim
To evaluate the R‐INJ‐04 soft‐tipped injector, a new injector with an integral round nozzle manufactured by Rayner Intraocular Lenses, England.
Methods
16 Rayner C‐flex intraocular lenses (IOLs; Rayner Intraocular lenses, England) ranging between +10 and +30 D (2 for each power) were tested. An ophthalmic viscoelastic device (Healon, AMO, Santa Ana, California, USA) was applied to the injectors. The IOLs were loaded according to the company injector's instructions for use and were injected into a Petri dish. After the injection, all the IOLs and nozzles were evaluated by gross (macroscopic) and microscopic analyses and photographed under a light microscope. One lens of each power and the cartridge used for the implantation were then sent for further analysis by scanning electron microscopy (SEM). The rest of the IOLs were tested for power and modulation transfer function (MTF).
Results
All the injections were successful. No damage to the IOLs or to the injectors was found by gross examination, light microscopy and SEM. No deposits were found on the IOL optical surfaces or haptics. Power and MTF analysis showed a close match with the original measurements.
Conclusion
Our results suggest that the R‐INJ‐04 soft‐tipped injector is safe for the implantation of the C‐flex IOL with power range from 10 to 30 D. No structural damage to the IOLs or to the injectors was found, and the lens power and light transmission properties were not damaged in any way by the injection process.
One of the most popular ways to implant a foldable intraocular lens (IOL) through a small incision is by using an injector system. These systems usually consist of two parts: the injector and the cartridge. There have been several reports of damage occurring to certain acrylic IOLs while using injector systems, probably during the implantation itself. The reported damage includes marks or scratches,1,2 stress fractures,3 cracks4 and tear lines.5
In a recent study,6 we found deposits on the posterior optical surface of the ACR6D SE IOL (Corneal, France) after implantation through a hexagonal cartridge. These deposits were not found when the same IOL was implanted through a round or oval cartridge.
The purpose of this study was to evaluate the R‐INJ‐04 soft‐tipped injector, a new round injector manufactured by Rayner Intraocular Lenses, England.
Methods
Sixteen C‐flex IOLs (Rayner Intraocular Lenses) ranging between 10 and 30 D (two for each power) were tested in this study. For insertion, an ophthalmic viscoelastic device (OVD; Healon, AMO, Santa Ana, California, USA) was applied to the nozzle and to both sides of the inner portion of the cartridge of the new R‐INJ‐04 soft‐tipped injector (Rayner Intraocular Lenses; fig 1). The amount of the OVD that was used for each IOL injection was evaluated subjectively between 1 and 5, and was documented. The IOLs were loaded according to the company's instructions for use. After 3 min in the cartridge, using gentle pressure on the inserter's plunger, the IOLs were injected into a Petri dish. After the injection, all the IOLs and cartridges were evaluated. Care was taken to minimise any manipulation of the IOL's optics with forceps or other grasping instruments by grasping the IOL's haptics. Gross (macroscopic) analyses of the IOLs and cartridges were performed. The lenses and cartridges were then evaluated microscopically and photographed under a light microscope. One lens of each power and the cartridge that was used for the implantation were then sent to the Electron Microscopy Center of the University of South Carolina (Columbia, South Carolina, USA) for further examination under a JEOL JSM 5410LV scanning electron microscope. In both light microscopy and SEM, cracks, scratches, marks, stress fracture, tear lines and deposits that were found on the IOL were defined as damage to the IOL. Stress lines, breaks and cracks were defined as damage to the cartridge nozzle. The rest of the IOLs were stored in a sealed vial with balanced salt solution and were sent to Rayner Intraocular Lenses for further analysis of the lens power and modulation transfer function (MTF) after implantation.
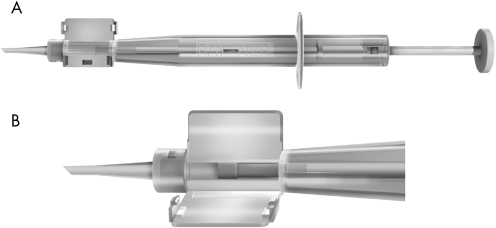
Figure 1 Company illustrations demonstrating the R‐INJ‐04 soft‐tipped injector (Rayner Intraocular Lenses, England). (A) Overview of the injector. (B) Magnification of the cartridge.
Results
All the injections were successful. No structural damage to the IOLs was found by gross examination, light microscopy and SEM, including cracks, scratches, marks, stress fracture and tear lines. No deposits were found on the IOL optical surfaces or haptics (fig 2). Normal remnants of OVD/balanced salt solution materials that were found on the IOL surfaces were easily removed with short rinsing of the IOLs. The cartridges remained complete with remnants of OVD. No damage to the cartridges was recorded, including stress lines breaks or cracks. The lenses' power and MTF measurement before and after injection were all within the ISO 11979‐2 standard tolerances. (ISO 11979 ophthalmic implants—intraocular lenses—part 2: optical properties and test methods.) The MTF measurement of IOLs after injections ranged from 0.48 to 0.6, with an average (SD) of 0.54 (0.037). The amount of the OVD that was used in each injection was evaluated subjectively and documented to range from 3 to 5, average (SD) 3.75 (0.62). The amount of the OVD that was used had no influence on the results or ease of the injections.
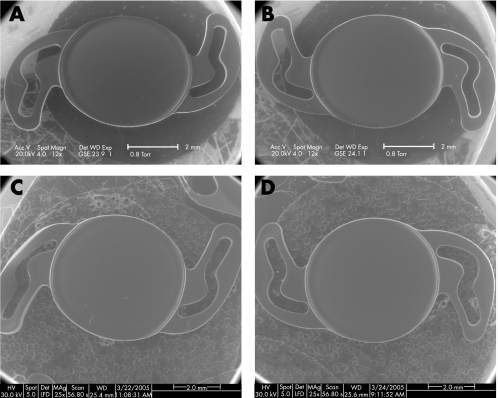
Figure 2 Scanning electron micrographs of one intraocular lens (IOL) after the injection compared with a control IOL that was not injected. (A) scanning electron microscopy (SEM) of the anterior surface of the C‐flex IOL after injection through the R‐INJ‐04 soft‐tipped injector showed no damage to the IOL anterior surface (original magnification ×12). (B) SEM of the posterior surface of the same IOL from fig 1A showing no damage to the IOL posterior surface (original magnification ×12). (C) SEM of the anterior surface of a control C‐flex IOL that was not injected showing an appearance similar to the injected IOL (original magnification ×25). (D) SEM of the posterior surface of the same IOL from fig 1C again showing an appearance similar to the injected IOL (original magnification ×25).
DISCUSSION
In this study, we demonstrated that the R‐INJ‐04 soft‐tipped injector is safe for the injection of the C‐flex intraocular lens with power range between 10 and 30 D. No structural damage to the IOLs or to the injectors was found after the injections.
In a previous study,6 we demonstrated that while using a hexagonal cartridge for the implantation of a different but similar hydrophilic acrylic IOL (ACR6D SE, Corneal, France), deposits were found on the IOL's posterior optical surface and the injector was found to have a major break line with several stress lines. Implantation of the same IOL through a round cartridge showed no damage or deposits. We determined the origin of the deposits on the IOL surface to be the inner part of the injector. We concluded that the mismatch between the hexagonal cartridge and the oval or round profile of the folded IOL increased the stress forces applied to the cartridge and thus caused extensive friction of the IOL's posterior surface (external surface of the IOL when folded) against the inner part of the cartridge. This could result in shaving or exfoliation, with deposit formations on the posterior surface of the IOL derived from the inner part of the cartridge surface.
The results of this study support our previous conclusion, which showed excellent results after implantation of the C‐flex IOL through a round cartridge. The R‐INJ‐04 soft‐tipped injector is a new round‐nozzle lens injector developed by Rayner Intraocular Lenses, England, for the implantation of the C and Superflex IOLs (fig 1). The plunger of the R‐INJ‐04 soft‐tipped injector is manufactured from a soft material. This allows the plunger to compress during the progression through the narrowing nozzle and by doing that the plunger fills the nozzle completely and tightly. Thus, if loaded correctly, no part of the lens can be trapped between the plunger and the nozzle, and also over‐ride of the plunger over the IOL is impossible (fig 3).
Figure 3 Gross photograph of the injection process of the C‐flex intraocular lens (IOL) through the R‐INJ‐04 soft‐tipped injector. The plunger is manufactured from a soft material. This allows it to compress during the progression through the narrowing nozzle and by doing that the plunger fills the nozzle completely and tightly. Thus, if loaded correctly, no part of the lens can be trapped between the plunger and the nozzle, and also over‐ride of the plunger over the IOL is impossible.
In conclusion, we demonstrated that the R‐INJ‐04 injector with a rounded nozzle is safe for the implantation of the C‐flex IOL.
Precis
In a laboratory study, we found the R‐INJ‐04 injector, a new round soft‐tipped injector, safe for the implantation of the C‐flex IOL.
Abbreviations
IOL - intraocular lens
MTF - modulation transfer function
OVD - ophthalmic viscoelastic device
SEM - scanning electron microscopy
Footnotes
Funding: This study was supported in part by an unrestricted grant from Rayner Intraocular Lenses, England.
Competing interests: The authors have no financial or proprietary interest in any product mentioned in this paper. DJA is a consultant to Rayner Intraocular lenses, England.
References
- 1.Milazzo S, Turut P, Blin H. Alterations to the AcrySof intraocular lens during folding. J Cataract Refract Surg 1996221351–1354. [DOI] [PubMed] [Google Scholar]
- 2.Vrabec M P, Syverud J C, Burgess C J. Forceps‐induced scratching of a foldable acrylic intraocular lens [letter]. Arch Ophthalmol 1996114777. [DOI] [PubMed] [Google Scholar]
- 3.Pfister D R. Stress fractures after folding an acrylic intraocular lens. Am J Ophthalmol 1996121572–574. [DOI] [PubMed] [Google Scholar]
- 4.Carlson K H, Johnson D W. Cracking of acrylic intraocular lenses during capsular bag insertion. Ophthalmic Surg Lasers 199526572–573. [PubMed] [Google Scholar]
- 5.Kohnen T, Magdowski G, Koch D D. Scanning electron microscopic analysis of foldable acrylic and hydrogel intraocular lenses. J Cataract Refract Surg 1996221342–1350. [DOI] [PubMed] [Google Scholar]
- 6.Kleinmann G, Marcovich A L, Apple D J.et al Linear deposits on the surfaces of intraocular lenses implanted through a hexagonal cartridge which mimic scratches/cracks on the lenses. Br J Ophthalmol 2005891474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]