Conjunctival intraepithelial neoplasia (CIN) has traditionally been managed with surgical excision, combined with cryotherapy, with a wide range of reported recurrence rates.1 In cases of CIN that are too extensive to perform a complete surgical excision or in cases in which the surgical margins are involved, ophthalmologists are now using adjunctive topical antineoplastic agents such as mitomycin C2,3 and interferon α‐2b (IFNα‐2b)4,5,6,7,8,9,10,11,12 in place of, or in combination with, repeat surgical excision. Although the toxicity associated with the topical ophthalmic use of mitomycin C is well recognised,13,14,15 IFNα‐2b has been reported not to cause ocular surface toxicity.4,5,6,7,8,9,10,11,12 We report a case of corneal toxicity, manifest as epithelial microcyst formation, associated with the use of topical IFNα‐2b.
Case report
A 64‐year‐old man with a history of biopsy‐proven CIN of the left eye presented to one of the authors (AJA) for evaluation. Twenty‐two years earlier, he had undergone a superficial keratectomy and excision of a papillomatous conjunctival lesion from the left eye. Six years before presentation he had undergone a second superficial keratectomy, and a limbal conjunctival biopsy 3 years later demonstrated epithelial squamous cell carcinoma in situ. The patient was treated with a 4‐week course of topical mitomycin C (0.02% initially, then 0.01%), which was discontinued secondary to poor tolerance.
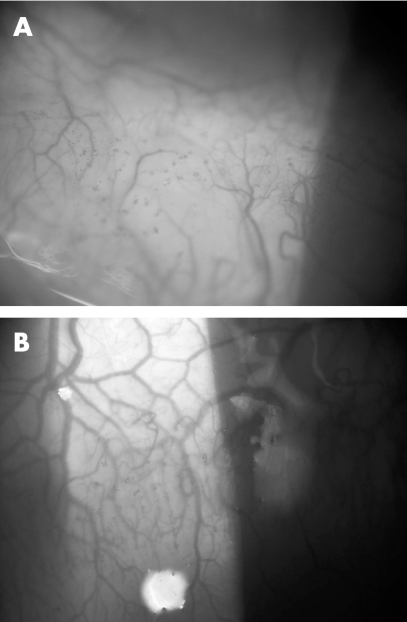
On presentation, the patient's visual acuity was limited to counting fingers in the left eye secondary to a dense cataract. Unilateral 360° micropannus and scattered punctate epithelial keratopathy (PEK) were noted in the cornea of the left eye. Several foci of fine papilliform vessels were noted in the nasal and limbal bulbar conjunctiva (figs 1A,B); biopsy specimens taken from these regions demonstrated marked atypia of the epithelial cells, consistent with CIN, extending to the edges of the submitted specimens.
Figure 1 Slit lamp photomicrographs demonstrating fine corkscrew vessels in the inferonasal bulbar (A) and superior limbal (B) conjunctiva of the left eye.
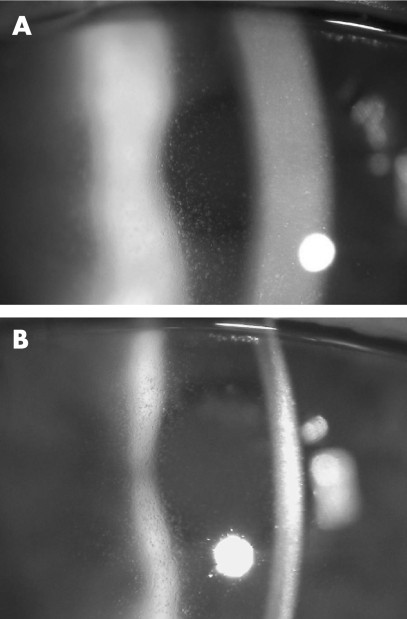
As the extensive conjunctival vascular abnormalities were too diffuse to perform a complete surgical excision, adjunctive topical treatment with mitomycin C was considered. However, given the previous poor tolerance of mitomycin C and concern about exacerbation of corneal limbal stem cell compromise secondary to the previous limbal keratectomies and mitomycin C‐associated toxicity, the patient was started on topical IFNα‐2b (1 million IU/ml; prepared from injectable powder mixed with preservative‐free normal saline) four times daily. Four weeks later, diffusely distributed, clear corneal epithelial microcysts were noted, prompting discontinuation of the topical interferon (figs 2A,B). Following an uncomplicated cataract extraction, the corrected visual acuity improved to 20/30, limited by central PEK and persistent epithelial microcysts. At 1 year after treatment with topical IFNα‐2b, the corneal epithelial microcysts were still present, as were the limbal papilliform vessels, although the patient declined additional therapy.
Figure 2 (A) Slit lamp photomicrograph demonstrating fine, diffuse, clear epithelial microcysts in the left cornea 4 weeks after instigating topical interferon α‐2b treatment. (B) Scattered punctuate epithelial staining in the slit lamp beam, overlying, but not necessarily corresponding to, the epithelial microcysts.
Comment
Interferons are a group of proteins that bind to surface receptors of target cells, triggering a cascade of intracellular antiviral and antitumour activities.7,10 Previous reports have shown topical IFNα‐2b, with or without subconjunctival IFNα‐2b, to be very effective in the treatment of primary and recurrent CIN.5,6,7,8,9,10,11,12,16,17 To the best of our knowledge, none of the 40 cases reported have documented associated corneal epithelial toxicity,5,6,7,8,9,10,11,12,16,17 although after 2 weeks of treatment, four times a day, one patient developed mild PEK, which resolved after discontinuation of the topical interferon.16 A transient follicular conjunctivitis has also been reported in five patients,7,8 presumed by one author reporting four of these five cases to be related to the vehicle used in the topical IFNα‐2b preparation,7 as no evidence of corneal or conjunctival epithelial toxicity was demonstrated previously in an animal model.4 As the topical IFNα‐2b drops utilised by the patient reported here were prepared using only preservative‐free normal saline, we may safely conclude that the observed corneal epithelial changes were not secondary to vehicle or preservative‐related toxicity.
The development of corneal epithelial microcysts in the case reported here is evidence of the ocular surface toxicity that may be seen in patients treated with topical IFNα‐2b. Corneal epithelial microcystic formation, identical to that noted in the patient reported here, has been reported with the use of systemic interferon treatment,18 and is a well‐recognised complication of the systemic administration of the antineoplastic agent cytarabine (Ara‐C).19,20 Corneal toxicity associated with high‐dose systemic cytarabine is thought to be secondary to the inhibition of DNA synthesis in the rapidly dividing basal corneal epithelial cells.20 Similarly, the antineoplastic actions of interferon involve immune‐enhancing properties as well as inhibition of cellular proliferation.21 An alternative mechanism that has been proposed to explain corneal epithelial microcyst formation in association with systemic interferon treatment is increased intercellular adhesion and altered corneal epithelial cell migration via an interferon‐mediated increased expression of intercellular adhesion molecule‐1.18 The development of the epithelial cysts several weeks after the initiation of topical interferon treatment, whether through inhibition of DNA synthesis, alteration of epithelial cell migration or another mechanism, indicates that IFNα‐2b‐related corneal epithelial cell toxicity is the most likely explanation for the origin of the microcysts. Ophthalmologists should be aware of the fact that ocular surface toxicity may be associated with topical IFNα‐2b treatment, and that it should be used judiciously in patients with corneal and conjunctival intraepithelial neoplasia.
Footnotes
Competing interests: None declared.
References
- 1.Tunc M, Char D H, Crawford B.et al Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. Br J Ophthalmol 19998398–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frucht‐Pery J, Rozenman Y, Pe'er J. Topical mitomycin‐C for partially excised conjunctival squamous cell carcinoma. Ophthalmology 2002109548–552. [DOI] [PubMed] [Google Scholar]
- 3.Rozenman Y, Frucht‐Pery J. Treatment of conjunctival intraepithelial neoplasia with topical drops of mitomycin C. Cornea 2000191–6. [DOI] [PubMed] [Google Scholar]
- 4.Smith M, Trousdale M D, Rao N A.et al Lack of toxicity of a topical recombinant interferon alpha. Cornea 1989858–61. [PubMed] [Google Scholar]
- 5.Maskin S L. Regression of limbal epithelial dysplasia with topical interferon. Arch Ophthalmol 19941121145–1146. [DOI] [PubMed] [Google Scholar]
- 6.Vann R R, Karp C L. Perilesional and topical interferon alfa‐2b for conjunctival and corneal neoplasia. Ophthalmology 199910691–97. [DOI] [PubMed] [Google Scholar]
- 7.Schechter B A, Schrier A, Nagler R S.et al Regression of presumed primary conjunctival and corneal intraepithelial neoplasia with topical interferon alpha‐2b. Cornea 2002216–11. [DOI] [PubMed] [Google Scholar]
- 8.Karp C L, Moore J K, Rosa R H., Jr Treatment of conjunctival and corneal intraepithelial neoplasia with topical interferon alpha‐2b. Ophthalmology 20011081093–1098. [DOI] [PubMed] [Google Scholar]
- 9.Hu F R, Wu M J, Kuo S H. Interferon treatment for corneolimbal squamous dysplasia. Am J Ophthalmol 1998125118–119. [DOI] [PubMed] [Google Scholar]
- 10.Boehm M D, Huang A J. Treatment of recurrent corneal and conjunctival intraepithelial neoplasia with topical interferon alfa 2b. Ophthalmology 20041111755–1761. [DOI] [PubMed] [Google Scholar]
- 11.Di Pascuale M A, Espana E M, Tseng S C. A case of conjunctiva‐cornea intraepithelial neoplasia successfully treated with topical mitomycin C and interferon alfa‐2b in cycles. Cornea 20042389–92. [DOI] [PubMed] [Google Scholar]
- 12.Holcombe D J, Lee G A. Topical interferon alfa‐2b for the treatment of recalcitrant ocular surface squamous neoplasia. Am J Ophthalmol 2006142568–571. [DOI] [PubMed] [Google Scholar]
- 13.Heigle T, Stulting R D, Palay D A. Treatment of recurrent conjunctival epithelial neoplasia with topical mitomycin C. Am J Ophthalmol 1997124397–399. [DOI] [PubMed] [Google Scholar]
- 14.Wu K, Hong S J, Huang H T.et al Toxic effects of mitomycin‐C on cultured corneal keratocytes and endothelial cells. J Ocul Pharmacol Ther 199915401–411. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Basti S. Corneoscleral, ciliary body, and vitreoretinal toxicity after excessive instillation of mitomycin C. Am J Ophthalmol 1992114503–504. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi A, Yoshita T, Uchiyama K.et al Successful management of conjunctival intraepithelial neoplasia by interferon alpha‐2b. Jpn J Ophthalmol 200246215–217. [DOI] [PubMed] [Google Scholar]
- 17.Schechter B A. Conjunctival intraepithelial neoplasia. Ophthalmology 19991061642–1643. [DOI] [PubMed] [Google Scholar]
- 18.Fracht H U, Harvey T J, Bennett T J. Transient corneal microcysts associated with interferon therapy. Cornea 200524480–481. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus H, Hartnett M E, Reed M D.et al Comparison of the prophylactic effects of 2‐deoxycytidine and prednisolone for high‐dose intravenous cytarabine‐induced keratitis. Am J Ophthalmol 1987104476–480. [DOI] [PubMed] [Google Scholar]
- 20.Hopen G, Mondino B J, Johnson B L.et al Corneal toxicity with systemic cytarabine. Am J Ophthalmol 198191500–504. [DOI] [PubMed] [Google Scholar]
- 21.Hertzog P J, Hwang S Y, Kola I. Role of interferons in the regulation of cell proliferation, differentiation, and development. Mol Reprod Dev 199439226–232. [DOI] [PubMed] [Google Scholar]