Heritable mutations in the RB1 gene cause an autosomal dominant condition resulting in retinoblastoma1,2 and an increased risk of malignancies including pineoblastoma, neuroblastoma, chondrosarcoma, rhabdomyosarcoma, glioma, leukaemia, sebaceous carcinoma, squamous cell carcinoma and cutaneous melanoma.3,4,5,6 Individuals with heritable retinoblastoma can undergo prenatal diagnosis followed by termination to avoid passing on the mutation to the next generation.7 Preimplantation genetic diagnosis (PGD) offers a means of achieving an unaffected pregnancy from the outset. IVF is required for PGD to allow cell biopsy from embryos for genetic testing. Embryos without the germline RB1 mutation are transferred to the mother for implantation and pregnancy.
Case study
A 24‐year‐old woman with bilateral retinoblastoma (RB1, OMIM#180200), had a de novo M708R mutation in RB1 and was referred for PGD. She had a one‐year‐old child with the mutation and also had had a miscarriage and two terminations of affected pregnancies.
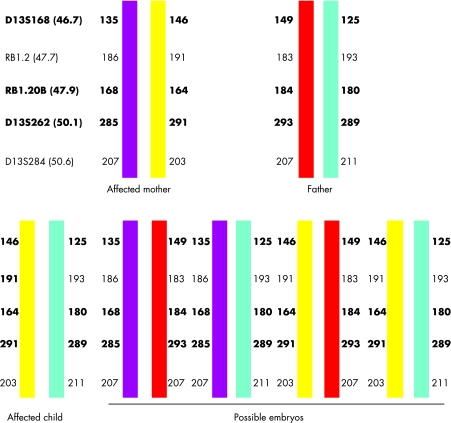
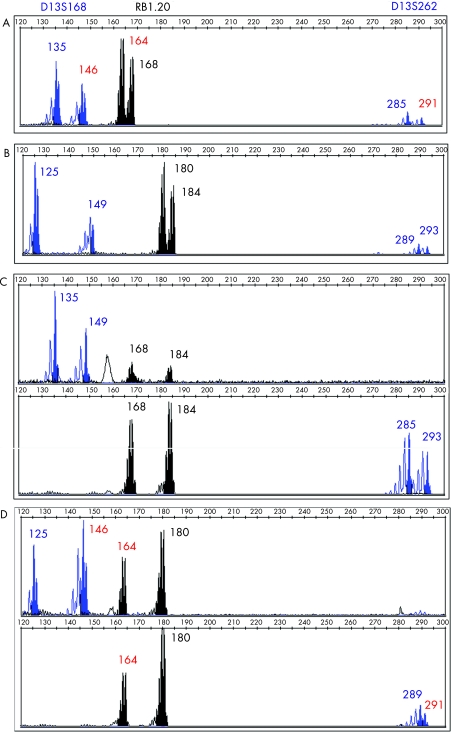
Following ovarian stimulation, 15 eggs were collected, of which 12 oocytes matured and eight fertilised normally 18–20 hours post‐intracytoplasmic sperm injection (ICSI). Seven embryos were suitable for biopsy on day 3. DNA from biopsied cells was amplified by PCR using fluorescently labelled intragenic (RBi20) and linked polymorphic micro‐satellite markers (D13S168 and D13S262). The PCR products were analysed using an ABI 3730 Prism. Three embryos (E4, E6 and E7) were diagnosed as carrying the maternal affected chromosome, while two embryos (E5 and E1) did not give clear results. Embryos E3 and E8 were diagnosed as unaffected. Figure 1 shows the haplotype for the family and figure 2 shows electrophoretograms of the parental DNA, a normal and an affected blastomere. All cell negatives and PCR negatives showed no DNA amplification.
Figure 1 Haplotype diagram of the family showing the allele sizes for each of the markers tested. The chromosome depicted in yellow carried the mutation as it was the one shared between the affected proband and her affected child. Using this information it was possible to predict from the haplotype which embryos carried the RB1 mutation. The markers shown in bold were used for PGD. The position of each of the markers on chromosome 13, in Mb, is given in parentheses.
Figure 2 Electrophoretograms from the DNA of proband (A), her partner (B), blastomeres from E8, normal embryo (C) and E6, embryo carrying the mutation (D) amplified with three markers: D13S168, RB1.20 and D13S262. Allelic length sizes are given in base pairs. The allelic sizes in red correspond to the maternal chromosome carrying the mutation.
On day 4 post‐insemination unaffected embryos E3 (5 cell) and E8 (morula), were transferred to the uterus. Six+ weeks gestation ultrasound showed two sacs, one with a viable fetal pole while the other was anembryonic. A healthy boy was delivered at 35/40 weeks. Here we report the first successful use of PGD for retinoblastoma in the UK.
Comment
This report highlights the feasibility of PGD for rare cancer predispositions. The indirect mutation detection strategy by haplotyping that we have reported may be applied to more than one family with different germline mutations provided DNA is available from other affected family members in order to identify the chromosome carrying the mutation. The difficulty in doing PGD for heritable retinoblastoma is that a large number of germline mutations have been identified8 and approximately half of these are de novo mutations.9 Separate PGD protocols need to be developed for each de novo germline mutation. PGD for cancer predisposition is therefore labour intensive and expensive, however, the value of PGD for retinoblastoma should be considered within the context of childhood manifestation of the disorder and a lifetime cancer risk in multiple organs.4,5,6 Inherited predisposition to retinoblastoma is very rare with a yearly UK incidence of approximately 20 cases.10 PGD could significantly reduce the incidence of this inherited disorder.
Footnotes
Competing interests: None.
References
- 1.Sparkes R S, Murphree A L, Lingua R W.et al Gene for hereditary retinoblastoma assigned to human chromosome‐13 by linkage to esterase‐D. Science 1983219971–973. [DOI] [PubMed] [Google Scholar]
- 2.Vogel F. Genetics of retinoblastoma. Hum Genet 1979521–54. [DOI] [PubMed] [Google Scholar]
- 3.Bader J L, Meadows A T, Zimmerman L E.et al Bilateral retinoblastoma with ectopic intra‐cranial retinoblastoma: trilateral retinoblastoma. Cancer Genet Cytogenet 19825203–213. [DOI] [PubMed] [Google Scholar]
- 4.Draper G J, Sanders B M, Kingston J E. 2nd primary neoplasms in patients with retinoblastoma. Br J Cancer 198653661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roarty J D, Mclean I W, Zimmerman L E. Incidence of 2nd neoplasms in patients with bilateral retinoblastoma. Ophthalmology 1988951583–1587. [DOI] [PubMed] [Google Scholar]
- 6.Traboulsi E I, Zimmerman L E, Manz H J. Cutaneous malignant‐melanoma in survivors of heritable retinoblastoma. Arch Ophthal 19881061059–1061. [DOI] [PubMed] [Google Scholar]
- 7.Overton C, Serhal P, Davies M. In: Harper JC, Delhanty JDA, Handyside AH, eds. Preimplantation Genetic Diagnosis. Chichester: Wiley, 2001123–140.
- 8.Valverde J R, Alonso J, Palacios I.et al RB1 gene mutation up‐date, a meta‐analysis based on 932 reported mutations available in a searchable database. BMC Genet 2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber J E, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol 200523276–292. [DOI] [PubMed] [Google Scholar]
- 10.Childhood Eye Cancer Trust http://www.chect.org.uk (accessed 4 Jun 2007)