Abstract
The cell cycle is the crucial process that leads to mitosis in all cell types. The dramatic redirectioning of many cellular processes during the cycle is known to involve ion channels, either changing their level of expression or their voltage dependence, as in the case of inward rectifiers. Here we describe the specific inhibition of heterologously expressed ionic channels at the onset of maturation in Xenopus oocytes. In cells expressing rat eag (R-eag) potassium channels, maturation induces a dramatic reduction in the current amplitude, which is almost complete in most cases. The key molecule in oocyte maturation, the mitosis-promoting factor (a complex of cyclin B and p34cdc2), is able to induce similar changes when injected into the oocytes.
Xenopus oocytes have been one of the main model systems used for the exploration of the cell cycle. Female germinal cells are arrested for an indefinite period of time in the G2 phase of the first meiotic cycle, until a hormonal stimulus induces progression of meiotic division. The completion of the second division renders a haploid egg ready for fertilization in a process known as maturation (1). The hormonal trigger for maturation is progesterone, acting on surface receptors (2); this starts a complex cascade in which the key step is the activation of mitosis-promoting factor (MPF) (3–5). The MPF complex consists of the catalytic subunit p34cdc2 under the control of the regulatory cyclin B. Cyclin B, synthesized de novo during G2 (6, 7), assembles with the existing p34cdc2, resulting in a complex, pre-MPF, which is kept inactive by specific phosphorylation at Thr-14 and Thr-15 of p34cdc2 (for a review, see ref. 8). At the transition from G2 to mitosis, the complex is suddenly activated and converted to MPF by dephosphorylation of these residues through cdc25 phosphatase, which itself becomes activated through Ser/Thr phosphorylation by MPF in an autocatalytic feedback loop (9). As of yet, the cascades both upstream and downstream of MPF activation remain only partially understood. Many of the substrates of MPF are still unidentified, but they include elongation factor 1-g, histone H1, p60v-src, p53, RNA polymerase II, and cyclin B itself. Nevertheless, the activation of MPF is the central regulatory point of the G2/M transition, since the injection of MPF alone into Xenopus oocytes is able to induce maturation.
During the cell cycle, depolarization of the membrane potential, elevated intracellular pH values, and fluctuations in the ionic composition of the cytoplasm have all been reported (for a review, see ref. 1). Changes in voltage-dependent ion currents during the cell cycle have also been described (10–14). Such changes could be induced at any point of the extensively branched regulatory cascade of the cell cycle, and the question as to whether they are a cause or a consequence of MPF activation remains unsolved. To our knowledge, no modification of any ion channel by MPF activation has been reported. We have studied the effects of both progesterone treatment and injection of MPF into Xenopus oocytes expressing R-eag (15) currents with electrophysiological techniques. We report that shortly after the activation of MPF, a known cloned ion channel is suppressed.
MATERIALS AND METHODS
cRNA encoding R-eag was prepared using a template with a T7 promoter following a standard protocol (16), and injection into Xenopus oocytes was performed as described (17). In brief, the oocytes were surgically extracted and then dissociated with a 2- to 3-h treatment with 28 mg/ml collagenase (Worthington) in calcium-free Barths medium containing 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, and 7.5 mM Tris·HCl (pH 7.4). The oocytes were then selected based on their size and clear differentiation between the light and dark sides. Only oocytes apparently in stages V and VI were injected. The oocytes were incubated in Barths medium [including 0.33 mM Ca(NO3)2 and 0.41 mM CaCl2] at 18°C.
The electrophysiological recordings were performed 1–7 days after injection, using a Turbo TEC-10CD amplifier (NPI Instruments, Tamm, Germany). The intracellular electrodes had resistances of 0.6–1 MΩ when filled with 2 M KCl. All of the records presented were leak subtracted on-line, using a P/n protocol. Acquisition and data analysis was achieved using the pulse-pulsefit software package (HEKA Electronics, Lambrecht/Pfalz, Germany). All recordings were performed in an external solution (NFR) containing 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM Hepes-NaOH (pH 7.2), with or without progesterone applied at the indicated concentrations. Progesterone was dissolved either 20 mM in dimethyl sulfoxide or 1 mM in ethanol, freshly added to the external medium, and then applied either 1 h before or continuously during the measurement. Monoclonal anti-cyclin B1 antibody (Santa Cruz Biotechnology) and control anti-mouse IgG (Sigma) were injected 15–60 min prior to the progesterone treatment. Active MPF (0.75 unit/μl) from starfish eggs was purchased from Promega; it was injected (≈50 nl) into oocytes during or immediately before the electrophysiological measurement.
RESULTS
Effects of Progesterone on the Current–Voltage (I–V) Relationship of R-eag.
To test whether R-eag was affected during the G2/M transition, we applied progesterone to oocytes expressing R-eag and compared the I–V relationships before and after the application of the hormone. Initially, the experiments were performed after a short (1-min) exposure to the progesterone stimulus. Under such conditions, channel behavior changed dramatically at times varying from 20 min to several hours after application of progesterone, depending on the batch of oocytes (Fig. 1). Regardless of the differences in the time required for channel modulation, all oocytes tested (n > 100) showed comparable effects.
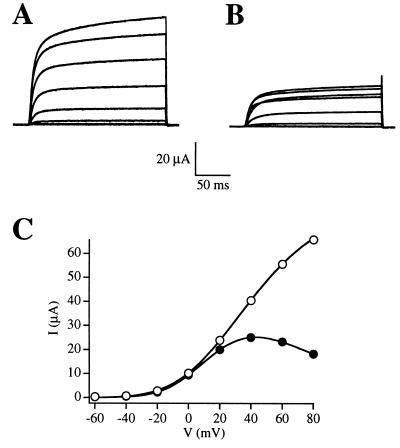
Figure 1.
Comparison of the shape and amplitude of raw current traces (A, B) and the I–V relationships (C) of R-eag before (A, open circles) and after (B, solid circles) the treatment with progesterone. Currents were elicited by depolarizing steps in Xenopus oocytes expressing R-eag mRNA, before (A) or during (B) treatment with 5 μg/ml progesterone. The depolarizations ranged from −60 to +80 mV in increments of 20 mV from a holding potential of −100 mV.
The first change that we observed was a voltage-dependent inhibition. Examples are given in Figs. 1B and 2B, where the current elicited at +80 mV was smaller than the current at +60 mV. This caused the I–V relationship to lose its linearity, as shown in Fig. 1C.
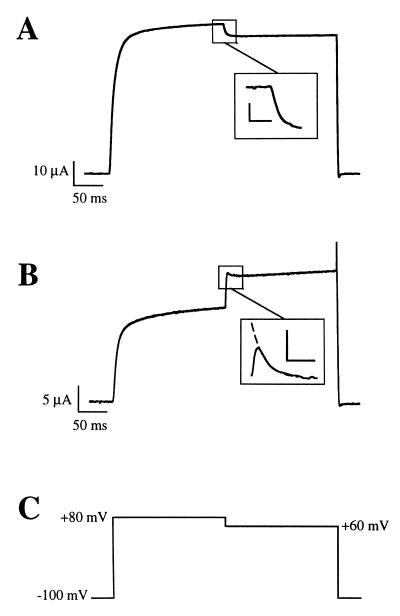
Figure 2.
Representative traces of the effect of a voltage step from +80 to +60 mV before (A) and 1 h after the application of 5 μg/ml progesterone (B). For these experiments, oocytes were held at −100 mV and the current was elicited by a depolarization to +80 mV. After 200 ms, the voltage was stepped to +60 mV, as depicted by the voltage template in C. The Inset of B shows the extrapolation to time 0 of the −20 mV step. The scale bars in the insets represent 2 μA and 10 ms (A) and 500 nA and 10 ms (B).
The presence of a voltage-dependent inhibition can also be demonstrated by different pulse protocols. The differences in amplitude at voltages where the maximal open probability has been achieved (+60 and +80 mV) should be seen easily in a single pulse containing both potentials. Before application of progesterone, the −20 mV step (from +80 to +60 mV) induced the expected exponential decay to a lower current amplitude (Fig. 2A and Inset). After progesterone treatment, however, a clear biphasic behavior was observed. On stepping to +60 mV, the current instantaneously increased before it relaxed to its steady state, which in virtually all cases was larger than at +80 mV (Fig. 2B). This resulted in a current “spike” at the beginning of the voltage step that was clearly observed in all experiments. This spike was used to monitor both the effectiveness of progesterone treatment and the degree of the voltage-dependent inhibition. It is interesting to point out here that the channel inhibition appears to be removed by the repolarizing pulse and that only then is it reestablished at the new potential.
The long time required to observe a response made comparison between oocytes before and after progesterone treatment difficult. For this reason, we used the development of the initial current peak (Fig. 2B, Inset) after the voltage step from +80 to +60 mV to quantify the action of the hormone. This initial increase in current amplitude should be proportional to the total amount of current inhibited, and it was estimated by extrapolating the exponential decay of the initial current peak to the moment the voltage was changed (see inset of Fig. 2B). This value was then taken as control current, and the difference between the steady-state and the control current, expressed as a percentage (of control), was used to estimate the degree of inhibition. Since the inhibition is likely not totally relieved during the 20 mV step, this value is an underestimation of the real inhibition. Pooled data from different batches of oocytes showed an inhibition of 27 ± 4% (mean ± SEM) (n = 14) after the progesterone treatment versus 4.8 ± 2.2% (n = 14; this reflects the decrease in driving force from +80 to +60 mV) before the treatment (Fig. 2 A and B). Interestingly, these changes are not a general property of all K+ channels, since no comparable effect of progesterone treatment was detected in oocytes expressing either Shaker H4 (18–20), Drosophila eag (21, 22), or Kv1.4 (23) channels (not shown).
Requirement of Cyclin B for the Action of Progesterone on R-eag.
A relevant question is to clarify the transduction pathway that leads to channel modification. Progesterone is known to increase the intracellular calcium concentration (1), and for this reason we tested whether either a transient or a sustained elevation of intracellular calcium could mimic the channel modulation. The induction of a rise in intracellular calcium by the addition of 10 mM caffeine (24) failed to induce modifications of the channel other than a homogeneous reduction of current amplitude (Fig. 3), as described previously by Stansfeld et al. (25). Upon washout of caffeine, the current traces were indistinguishable from control traces.
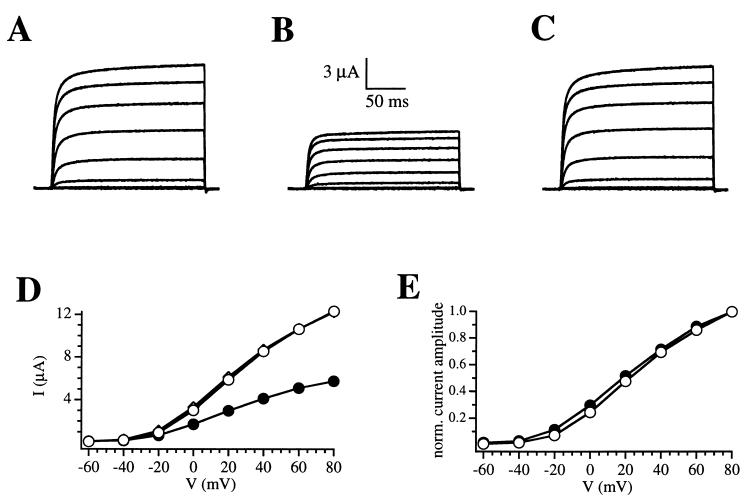
Figure 3.
Effect of caffeine-induced calcium release on R-eag current amplitude. (A) Control traces. During the application of 10 mM caffeine (B), the current is reduced at all voltages. After washout (C), the amplitude readily recovers and remains stable for the rest of the experiment. (D) I–V relationships corresponding to control traces (open circles), 10 mM caffeine (solid circles), and washout of the drug (diamonds). The data correspond to A, B, and C, respectively. The washout I–V plot is virtually overlapping the control oneplot. (E) I–V plots from D (records A and B) have been scaled to show that the current amplitude is homogeneously reduced throughout the voltage axis by the action of caffeine (symbols are as in D).
Naturally occurring polyamines, such as spermine and spermidine, have been shown to effectively block potassium channels, especially inward rectifiers (26, 27). For this reason, we tested the effect of the injection of spermine while recording in two-electrode voltage clamp and the effect of the addition to the intracellular side of inside-out patches of the most potent of the two amines, spermine. In these experiments, concentrations up to 5 μM had no effect on R-eag current amplitude nor on the shape of the I–V relationship.
Another possibility for the transduction of the signal is the activation of a protein kinase. To examine whether a kinase was indeed involved, we attempted to block the effect of progesterone by the addition of protein kinase inhibitors. We found that all kinase inhibitors tested (50 μM H7, 3 μM GF109203X, 50 μM genistein) effectively blocked the modification of channel behavior (data not shown).
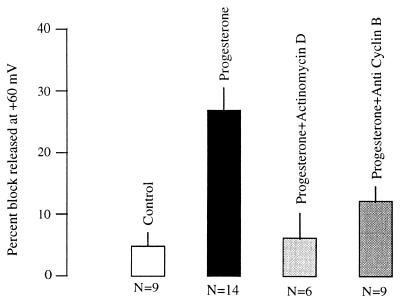
The fact that different kinases are necessary for the inhibition of the current suggested to us that the action of progesterone on R-eag does not rely on a single transduction route and that the confluence point into the cell cycle (i.e., the MPF activation) could be necessary for channel modification. If this were the case, it should be possible to inhibit the action of progesterone by blocking the activation of MPF, either by inhibiting de novo synthesis of cyclin B or blocking the protein once it has been synthesized. To test this, we used actinomycin D, an RNA synthesis inhibitor. As seen in Fig. 4, incubation with 10 μg/ml actinomycin D significantly reduced the action of progesterone on R-eag, thus indicating that newly synthesized RNA was necessary for progesterone to modify the channel. One of the possible proteins whose synthesis is required for the modification of the channel is cyclin B, which is synthesized precisely at this point of the cycle. To check whether cyclin B was involved in the action of progesterone, we tested whether antibodies against cyclin B1 could block progesterone action on the channel. The injection of a monoclonal antibody (10 ng) against human cyclin B1 before the progesterone treatment was sufficient to block the effect, as shown in Fig. 4, while the injection of anti-mouse Fc IgG produced no effect.
Figure 4.
Effect of progesterone (5 μg/ml) on the development of voltage-dependent inhibition of R-eag currents, and inhibition of the effect of the hormone in oocytes preincubated with 10 μg/ml actinomycin D or injected with 10 ng anti human cyclin B1 antibodies 30 min before progesterone application.
Effects of the injection of MPF into Xenopus Oocytes.
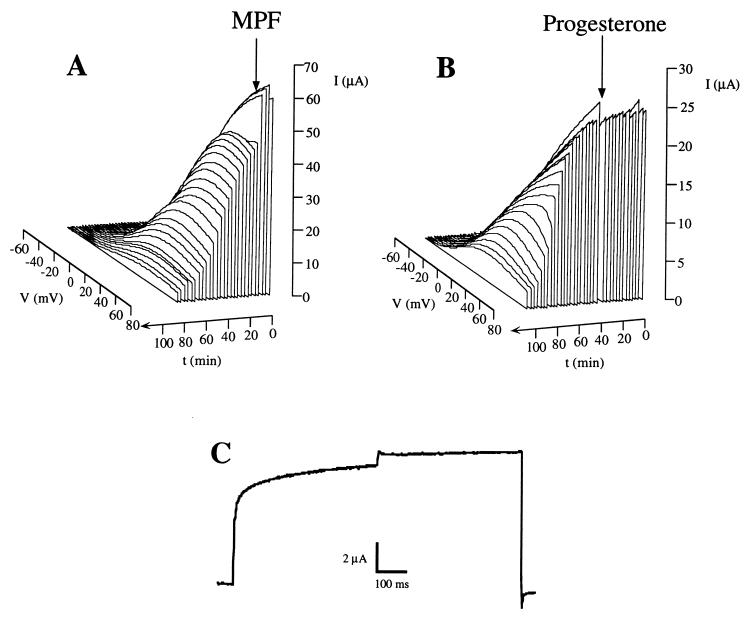
To further test the hypothesis that MPF activation was responsible for the modulation of the channel, we monitored R-eag currents expressed in oocytes after the injection of active MPF. In these experiments, the main effect observed was a dramatic reduction in current amplitude at all voltages (Fig. 5A). For comparison, a similar time course is shown in Fig. 5B for the application of progesterone. The main difference observed between the two treatments was the much faster development of the current reduction after MPF injection. In many cases, the voltage-dependent inhibition was evident only during the first minutes (Fig. 5C). After this time, the inhibition was apparent at all the voltages positive to the activation threshold of the channel, resulting in a strong reduction in current amplitude at all potentials. This supports the idea that the effect of MPF is the down-regulation of the channel activity via a voltage-dependent inhibition.
Figure 5.
Representation of the time course of the currents after the injection of MPF (A) and the application of progesterone (20 μg/ml) (B). The arrows indicate the time of injection or application. Note that for clarity, the time axis has been depicted such that it increaseds toward the front of the figure. (C) Initial effect produced by MPF injection, in an experiment identical to those depicted in Fig. 2 for progesterone treatment.
Upon closer examination of the data, two populations of oocytes could be distinguished on the basis of their response to the injection of MPF. In 22 out of 27 oocytes, the inhibition was found to be 97 ± 0.7% of the control current, almost a complete suppression. In the remaining five oocytes, however, the inhibition was weaker but still significant (49 ± 7%). A possible explanation for this difference was the difficulty in distinguishing oocytes from stages VI, V, and the end of stage IV by visual examination. Only in the oocytes of stage VI, and possibly stage V, can the injected MPF activate endogenous pre-MPF through the autocatalytic feedback loop (9). For this reason, the action of injected MPF is expected to be much faster and more complete in these stages.
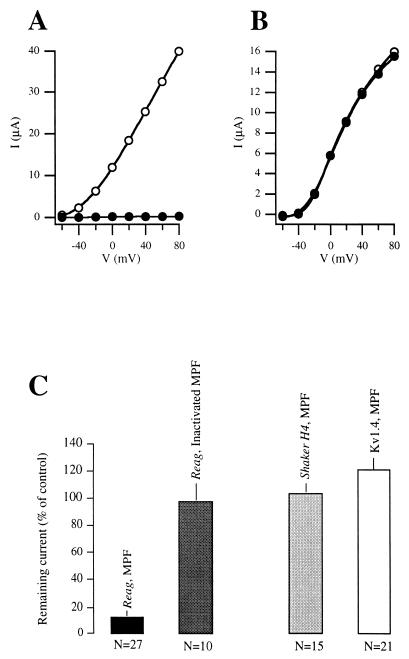
No significant effect was observed after the injection of heat-inactivated enzyme (n = 11), as shown in Fig. 6. In oocytes taken from the same donor frogs, the injection of MPF did not evoke significant inhibition of other K+ channels. In Shaker H4 (Fig. 6B), the remaining current was 104 ± 6% (n = 15), while in Kv1.4, the value obtained was 121 ± 8% (n = 21) of the control current amplitude (Fig. 6). This again reinforces the fact that the effect of MPF is specific for R-eag and not a general property of potassium channels.
Figure 6.
Current-voltage relationships in oocytes expressing R-eag (A) or Shaker H4 (B) channels, before (open circles) and after the injection of MPF (closed circles). In B, both curves are overlapping. (C) Reduction of current amplitude induced by the injection of MPF, or heat-inactivated MPF, into Xenopus oocytes expressing R-eag, or MPF into oocytes expressing either Shaker H4 or Kv1.4 channels.
DISCUSSION
Our experiments demonstrate a novel modulatory effect occurring downstream of the activation of MPF on a cloned heterologously expressed ion channel. Our results suggest a new mechanism for controlling channel activity during the cell cycle. In this respect, it is important to note that not all potassium channels undergo a similar regulation. Changes in the behavior of several channels have already been observed during cell cycle (10–14). This is the first case a channel with a defined molecular entity has been shown to be “cell cycle-sensitive.” Moreover, we show that MPF is sufficient to suppress channel activity. This allows us to concentrate on only one of the many signaling routes implicated in the crucial process of the cell cycle.
It has recently been shown that an inwardly rectifying K+ channel, which shows features that closely resemble those of HERG (a human member of the eag family) (28–30), is regulated in its voltage dependence of activation during the cell cycle, and that this directly influences the resting potential of the cells (14). Our results are consistent with these observations, showing that another member of the same family, a delayed rectifier, can be modulated by the action of MPF in an heterologous system. These results give some insight into the possible molecular mechanisms underlying the control of eag channels during cell cycle and suggest a role for the channels of this family in this process.
There are two relevant questions that will require further work for clarification. First, is the down-regulation of R-eag a direct catalytic effect of MPF? Second, what is the physiological role for the suppression of the channel function?
MPF could directly phosphorylate the channel protein, since the primary sequence of R-eag shows four consensus sequences for MPF substrates. On the other hand, the lag between progesterone application and current reduction disappears when MPF is injected into the oocytes, and the effect is then virtually instantaneous. For this reason, we favor the hypothesis that R-eag is a direct substrate for MPF. In vitro experiments are currently being performed in our laboratory to clarify this point further. It also remains to be investigated whether other cyclin-dependent kinases are able to modify this channel.
The physiological role for R-eag modulation is certainly more difficult to clarify, but several suggestive hypotheses can be made. Overexpression of R-eag channels in Xenopus oocytes resulted in a 40-mV shift of the resting potential of the oocytes, from approximately −30 to −70 mV (not shown). After the treatment with progesterone or injection of MPF, the oocytes consistently exhibited a considerably more positive resting potential (again approximately −30 mV). This observation clearly suggests a role for the suppression of this delayed rectifier in the native system [i.e., the depolarization of the resting potential, which in fact is observed during mitosis (1)]. It is possible that native R-eag channels undergo comparable changes in the cells where they are endogenously expressed, since MPF is a ubiquitous intracellular factor triggering the G2/M-transition in the cell cycle of different organisms ranging from yeast to human (31).
Acknowledgments
We wish to thank Prof. O. Pongs for the generous gift of R-eag clone, Dr. Martin Stocker for preparing the RNA, Dr. S. Ramos for the anti-cyclin B antibody, the group of Prof. Hilschmann for the anti-mouse Fc antibody, Dr. J. M. Martín for the help with the in vitro synthesis, Susanne Voigt for technical assistance, Drs. F. Barros and P. de la Peña for valuable help and support, and Drs. G. Busch, R. H. Chow, M. Krause, A. B. Parekh, O. Paulsen, P. Pedarzani, M. Stocker, and H. Terlau for critical reading of the manuscript. During this work, L.A.P. was supported by a “Contrato de Incorporación” from Universidad de Oviedo and Dirección General de Investigación Cientifica y Ténica.
Footnotes
Abbreviations: MPF, mitosis-promoting factor; I–V, current–voltage.
References
- 1.Hausen P, Riebesell M. The Early Development of Xenopus laevis. Berlin: Springer; 1990. pp. 15–18. [Google Scholar]
- 2.Maller J L. Biochemistry. 1990;29:3157–3166. doi: 10.1021/bi00465a001. [DOI] [PubMed] [Google Scholar]
- 3.Lee M, Nurse P. Nature (London) 1987;327:31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- 4.King R W, Jackson P K, Kirschner M W. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 5.Nurse P. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 6.Murray A W, Solomon M J, Kirschner M W. Nature (London) 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 7.Murray A W, Kirschner M W. Nature (London) 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 8.Dunphy W G. Trends Cell Biol. 1994;4:202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann I, Clarke P R, Marcote M J, Karsenti E, Draetta G. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day M L, Pickering S J, Johnson M H, Cook D I. Nature (London) 1993;365:560–562. doi: 10.1038/365560a0. [DOI] [PubMed] [Google Scholar]
- 11.Block M L, Moody W J. Science. 1990;247:1090–1092. doi: 10.1126/science.2309122. [DOI] [PubMed] [Google Scholar]
- 12.Coombs J L, Villaz M, Moody W J. Dev Biol. 1992;153:272–282. doi: 10.1016/0012-1606(92)90112-t. [DOI] [PubMed] [Google Scholar]
- 13.Gurantz D, Ribera A B, Spitzer N C. J Neurosci. 1996;16:3287–3295. doi: 10.1523/JNEUROSCI.16-10-03287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. J Physiol (London) 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig J, Terlau H, Wunder F, Brüggemann A, Pardo L A, Marquardt A, Stühmer W, Pongs O. EMBO J. 1994;13:4451–4458. doi: 10.1002/j.1460-2075.1994.tb06767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg P A, Melton D A. Methods Enzymol. 1987;155:421–430. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- 17.Stühmer W. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- 18.Kamb A, Iverson L E, Tanouye M A. Cell. 1987;50:405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- 19.Papazian D M, Schwarz T L, Tempel B L, Jan Y N, Jan L Y. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 20.Pongs O, Kecskemethy N, Müller R, Krah-Jentgens I, Baumann A, Kiltz H H, Canal I, Llamazares S, Ferrús A. EMBO J. 1988;7:7087–7096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warmke J, Drysdale R, Ganetzky B. Science. 1991;252:1562–1564. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 22.Brüggemann A, Pardo L A, Stühmer W, Pongs O. Nature (London) 1993;365:445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- 23.Stühmer W, Ruppersberg J P, Schröter K H, Sakmann B, Stocker M, Giese K P, Perschke A, Baumann A, Pongs O. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathanson M H, Moyer M S, Burgstahler A D, O’Carroll A-M, Brownstein M J, Lolait S J. J Biol Chem. 1992;267:23282–23289. [PubMed] [Google Scholar]
- 25.Stansfeld C E, Röper J, Ludwig J, Weseloh R M, Marsh S J, Brown D A, Pongs O. Proc Natl Acad Sci USA. 1996;93:9910–9914. doi: 10.1073/pnas.93.18.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ficker E, Taglialatela M, Wible B A, Henley C M, Brown A M. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 27.Fakler B, Brändle U, Glowatzki E, Weidemann S, Zenner H-P, Ruppersberg J P. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti M C, Jiang C, Curran M E, Keating M T. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 29.Trudeau M C, Warmke J W, Ganetzky B, Robertson G A. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 30.Smith P L, Baukrowitz T, Yellen G. Nature (London) 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 31.Nurse P. Nature (London) 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]