Abstract
Aim
To investigate the retinal toxicity of bevacizumab in co‐application with a commercially available recombinant tissue plasminogen activator (rt‐PA), and to facilitate a new therapeutic concept in the treatment of massive subretinal haemorrhage caused by neovascular age‐related macular degeneration (AMD).
Methods
Isolated bovine retinas were perfused with an oxygen‐preincubated nutrient solution. The electroretinogram (ERG) was recorded as a transretinal potential using Ag/AgCl electrodes. Bevacizumab (0.25 mg/ml) and rt‐PA (20 μg/ml) were added to the nutrient solution for 45 min. Thereafter, the retina was reperfused for 60 min with normal nutrient solution. Similarly, the effects of rt‐PA (20 μg/ml, 60 μg/ml and 200 μg/ml) on the a‐ and b‐wave amplitudes were investigated. The percentages of a‐ and b‐wave reduction during application and at washout were calculated.
Results
During application of bevacizumab (0.25 mg/ml) in co‐application with 20 μg/ml (rt‐PA), the ERG amplitudes remained stable. The concentrations of rt‐PA alone (20 μg/ml and 60 μg/ml) did not induce significant reduction of the b‐wave amplitude. In addition, 20 μg/ml rt‐PA did not alter the a‐wave amplitude. However, 60 μg/ml rt‐PA caused a slight but significant reduction of the a‐wave amplitude. A full recovery was detected for both concentrations during the washout. At the highest tested concentration of 200 μg/ml rt‐PA, a significant reduction of the a‐ and b‐wave amplitudes was provoked during the exposure. The reduction of ERG amplitudes remained irreversible during the washout.
Conclusion
The present study suggests that a subretinal injection of 20 µg/ml rt‐PA in co‐application with bevacizumab (0.25 mg/ml) for the treatment of massive subretinal haemorrhage seems possible. This is a safety study. Therefore, we did not test the clinical effectiveness of this combined treatment.
Keywords: age‐related macular degeneration, bevacizumab, recombinant tissue plasminogen activator, electroretinogram, retinal toxicity
Introduction
Age‐related macular degeneration (AMD) is the leading cause of central vision loss in the aged population of the Western world.1 The neovascular form of the disease is characterized by the growth of a choroidal neovascularization (CNV). In particular, CNVs associated with a massive subretinal haemorrhage are correlated with a dramatic loss in vision, inducing irreversible damage of the neurosensory retina.2,3,4,5
Pars plana vitrectomy, subretinal tissue‐plasminogen activator (rt‐PA) injection and air‐fluid exchange are techniques that have been used effectively to displace submacular haemorrhage.6 Most patients have improved vision soon after the haemorrhage is removed from the subretinal space. However, visual acuity frequently deteriorates due to progression of the underlying pathology of the macula.7
Although the exact mechanisms of CNV are still not completely clarified, previous studies indicate that an imbalance between angiogenic and antiangiogenic factors may play an important role, in which vascular endothelial growth factor (VEGF), as an angiogenic factor, plays a central role in the development of CNV.8,9
Recently, new therapeutic agents have been introduced for the treatment of exudative AMD targeting VEGF. One of these new agents is bevacizumab (AvastinTM).10,11 The recombinant humanized anti‐VEGF antibody bevacizumab was shown in vitro to inhibit VEGF‐induced cell proliferation, survival, migration and tissue‐factor production, and recent studies proposed its application in the treatment of exudative AMD.12
Inhibiting the effects of VEGF, angiogenesis and vascular permeability makes bevacizumab a promising candidate for intraocular co‐application with rt‐PA in the treatment of exudative AMD with a massive subretinal haemorrhage, potentially reducing the risk of CNV progression or recurrence.
No data about the toxic side effects of bevacizumab in co‐application with rt‐PA on retinal function are available, when used at clinically relevant therapeutic concentrations. The present study was designed to investigate the effects of bevacizumab and rt‐PA on the parameters of the electroretinogram (ERG) using the isolated perfused vertebrate retina technique, an electrophysiological in vitro technique for the evaluation of retinal toxicity.13,14
Materials and methods
Materials
Aspartate, glucose and other chemicals were obtained from Merck at pro‐analysis grade. Commercially available rt‐PA (10 mg Alteplase, (Actilyse®)) was purchased from Boehringer Ingelheim (Germany) and was dissolved in 10 ml of a vehicle containing 348.4 mg arginine, 107.2 mg H3PO4, 1 mg polysorbate 80 and water for injection.
Bevacizumab (AvastinTM) was purchased from Roche Pharma (Switzerland). Bevazicumab was dissolved in 4 ml of a vehicle containing 240 mg α,α‐trehalose × 2H2O, 23.2 mg NaH2PO4 × H2O, 4.8 mg Na2HPO4, 1.6 mg polysorbate 20 and water for injection. The dilutions with dissolved rt‐PA and bevacizumab were used in a final concentration of 1 mg/ml and 25 mg/ml, respectively, as a stock solution. The stock solutions were stable when stored at 4°C.
A purified sheep anti‐bovine IgG was purchased from Immunology Consultants Laboratory Inc. (USA). The anti‐bovine IgG was dialysed with the nutrient solution and used as a control.
Methods
Superfused vertebrate retina assay
Bovine eyes were obtained directly post‐mortem and were transported in darkness in a serum‐free standard medium containing 120 mM NaCl, 2 mM KCl, 0.1 mM MgCl2, 0.15 mM CaCl2, 1.5 mM NaH2PO4, 13.5 mM Na2HPO4 and 5 mM glucose. The preparation was performed as described recently.13,15,16 For each experiment, a new isolated retina was used.
The ERG was recorded in the surrounding nutrient medium via two silver/silver‐chloride electrodes on either side of the retina. The recording chamber containing a piece of retina was placed in an electrically and optically insulated box. The perfusion velocity was controlled by a roller pump and set to 1 ml/min. The temperature was kept constant at 30°C. The perfusing medium was pre‐equilibrated and saturated with oxygen. The retina was dark‐adapted and the ERG was elicited at intervals of 5 min using a 1‐Hz single white xenon flash for stimulation. The flash intensity was set to 6.3 mlx at the retinal surface using calibrated neutral density filters (Kodak Wratten Filter).
The duration of light stimulation was 10 microseconds, controlled by a timer (Photopic Stimulator PS33 Plus; Grass, Warwick, RI). The ERG was filtered and amplified (100‐Hz high pass filter, 50‐hz notch filter, 100 000 × amplification) using a Grass RPS312RM Amplifier. The data were processed and converted with an analog‐to‐digital data acquisition board (PCI‐MIO‐16XE‐50; National Instruments, Austin, TX) in a desktop computer (PC compatible).
The retina was superfused with the serum‐free nutrient solution and stimulated repeatedly until stable b‐wave amplitudes were recorded. Bevacizumab at a concentration of 0.25 mg/ml and rt‐PA at a concentration of 20 μg/ml (n = 6) were added to the nutrient solution for 45 min and responses were recorded for 45 min. Thereafter, the retina was reperfused for 60 min with normal nutrient solution.
In our surgical setting, vitrectomy was combined with a subretinal injection of 20 μg/ml tissue plasminogen activator (rt‐PA) in the case of a subretinal haemorrhage (SRH) caused by neovascular AMD. According to our setting, the following concentrations of rt‐PA were selected and, in an analogous manner, the effects of rt‐PA (20 μg/ml [n = 6], 60 µg/ml [n = 6] and 200 μg/ml [n = 4]) alone were investigated on the b‐wave amplitude.
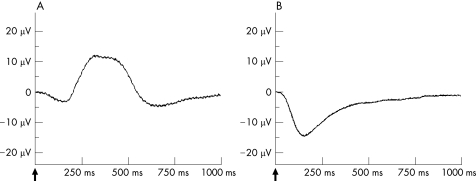
After recording stable ERG amplitudes, the retina was exposed to the indicated concentrations of rt‐PA for 45 min. Thereafter, perfusion with standard solution was resumed for another 60 min to observe the b‐wave recovery. The b‐wave amplitude was measured from the trough of the a‐wave to the peak of the b‐wave (fig. 1a).
Figure 1 The ERG from the isolated perfused bovine retina. A: The b‐wave is dominant in the ERG of the isolated perfused bovine retina under scotopic light conditions. It results from a 10‐microsecond light stimulus at a light intensity of 6.3 mlx at scotopic lighting conditions. B: The a‐wave is dominant in the ERG of the isolated perfused bovine retina after blocking the b‐wave by 1 mM aspartate to the nutrient solution. The a‐wave was generated by using a 10‐microsecond light stimulus of 6.3 mlx at scotopic lighting conditions.
To investigate the effects of bevacizumab on the photoreceptors under scotopic conditions (6.3 mlx flash light intensity), the b‐wave was suppressed by adding 1 mM aspartate to the nutrient solution. Under these conditions, the influence of bevacizumab on the photoreceptor potential P III was analysed. Aspartate is an inhibitor of synaptic transmission at the level of the first retinal synapse and enables the recording of unmasked photoreceptor potential P III by abolishing the b‐wave (fig. 1b).17 We recorded stable photoreceptor potential P III for 30 min. Thereafter, bevacizumab in co‐application with rt‐PA was added at the same concentrations to the aspartate‐containing nutrient solution for 45 min (n = 6). The changes of the a‐wave amplitude during the application of bevacizumab and rt‐PA were recorded and the recovery was followed up for 60 min under perfusion with the aspartate‐containing nutrient solution. The effects of rt‐PA alone on the photoreceptor potential were also tested at concentrations of 20 μg/ml (n = 6), 60 μg/ml (n = 6) and 200 μg/ml (n = 4), using the same experimental set‐up.
Additionally, the effects of the vehicle were tested on the a‐ and b‐wave amplitudes. The dilution without the active agent rt‐PA was used as a control. Equivalent amounts of the solvent carrier were applied to the nutrient solution for 45 min under the same conditions as during the testing of 20 μg/ml rt‐PA. Subsequently, the a‐ and b‐wave recovery was observed for 60 min (for both test series, n = 5).
We also included an experiment with a sheep anti‐bovine IgG as a negative control for the test series bevacizumab in co‐application with rt‐PA. The sheep anti‐bovine IgG was dialysed with the nutrient solution and we tested the effects on the b‐wave amplitude at the same final concentration of 0.25 mg/ml (n = 1).
Data analysis
The percentage reduction of the a‐wave and b‐wave amplitude was calculated before and after application of bevacizumab and rt‐PA. The a‐ and b‐wave recovery was compared with the a‐ and b‐wave amplitude before application of bevacizumab. For the statistical analysis, the software “JMP Version 6.0.3” was used. Significance was estimated by a multifactorial analysis of variance and levels of p⩽0.05 were considered as statistically significant.
Results
The perfusion of the isolated bovine retina was performed under stable environmental conditions. Osmotic pressure, temperature and pO2 remained unchanged during perfusion. In our experimental setting, stable ERG amplitudes were reached within 2 hours perfusion and the superfused retinal preparations responded constantly to light stimulation for more than 10 hours.13
Bevacizumab was effective in the treatment of neovascular AMD and macular oedema at a concentration of 1.25 mg per human eye after intravitreal injection.10,18 Therefore, based on approximately 5 ml of the vitreous humor, the average intraocular concentration of bevacizumab was calculated to be 0.25 mg/ml.
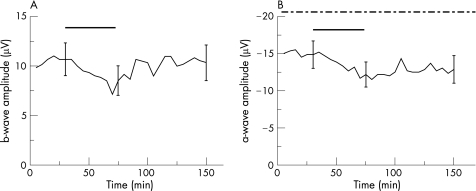
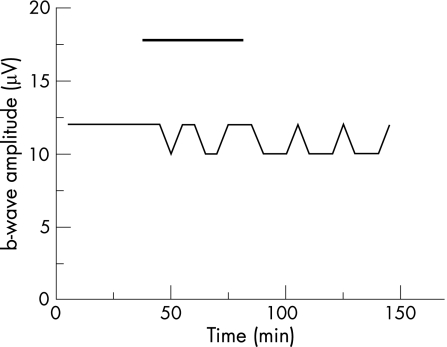
Accordingly, we studied the effects of bevacizumab on the parameters of the ERG at a concentration of 0.25 mg/ml in co‐application with 20 μg/ml rt‐PA. During the application of bevacizumab and rt‐PA, only a slight but not significant reduction of the b‐wave amplitude of 14.2% was found during the exposure time (p = 0.37, fig. 2a). Furthermore, after incubation, the b‐wave amplitude remained stable throughout the washout with the standard solution, showing no significant difference of b‐wave amplitude before and after application of bevacizumab and rt‐PA (p = 0.80; fig. 2a).
Figure 2 Effects of bevacizumab in co‐application with rt‐PA on the ERG of the isolated perfused bovine retina. The average of the representative drug series (both test series, n = 6). The black horizontal bar marks the time of combined bevacizumab (0.25 mg/ml) and rt‐PA (20 μg/ml) application. The dash‐dotted line in B marks the time of the additional aspartate application (1 mM). Three representative standard deviations for each drug series are given.
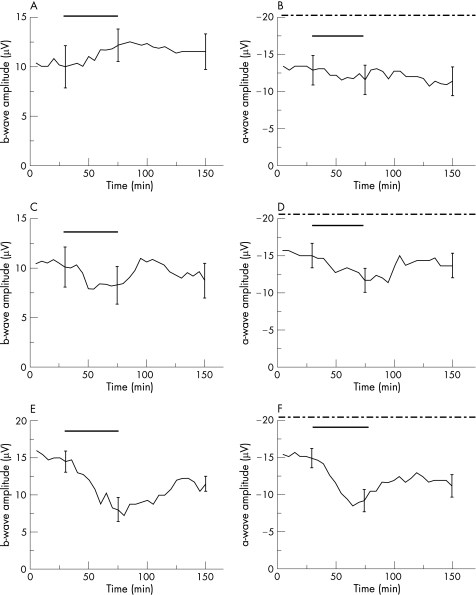
The separated effects of rt‐PA on the b‐wave amplitude were also tested at the following concentrations: 20 μg/ml, 60 μg/ml and 200 μg/ml. During exposure of 20 μg/ml rt‐PA, a slight increase of 20.53% of the b‐wave could be observed, which did not reach significance level (p = 0.10; fig. 3a). At 60 μg/ml rt‐PA, the applied drug provoked a reduction of the b‐wave amplitude of 27.9%, which was not significant (p = 0.25; fig. 3c). For both concentrations of rt‐PA, the b‐wave amplitude recovered during the washout, reaching the mean value of b‐wave amplitudes before the application of rt‐PA (fig. 3a and c).
Figure 3 Effects of rt‐PA on the ERG of the isolated perfused bovine retina. The average of representative drug series. The black horizontal bar marks the time of rt‐PA application. The dash‐dotted line in B, D and F marks the time of aspartate co‐application (1 mM). The rt‐PA concentrations: in A and B, a rt‐PA concentration of 20 μg/ml was used (n = 6); in C and D, a rt‐PA concentration of 60 μg/ml was tested (n = 6); in E (n = 4) and F (n = 4), a rt‐PA concentration of 200 μg/ml was investigated. Three representative standard deviations for each drug series are given.
In contrast, the highest tested concentration of 200 μg/ml rt‐PA provoked a pronounced reduction of the b‐wave amplitude of 44.64% at the end of the exposure period (p<0.01). A b‐wave recovery was not detected and the b‐wave amplitude remained impaired during the washout (p<0.01; fig. 3e).
To evaluate the influence of bevacizumab (0.25 mg/ml) in co‐application with rt‐PA (20 μg/ml) on the a‐wave amplitude, we investigated the effects of both therapeutic substances on the photoreceptor potential P III using aspartate.17 After the addition of 1 mM aspartate, the b‐wave amplitude was reduced continuously and unmasked photoreceptor potential could be recorded. The concentrations of bevacizumab and rt‐PA were added to the nutrient solution containing aspartate for 45 min. The washout for the a‐wave recovery was comparably limited to 60 min (fig. 2b). The a‐wave amplitude was changed by bevacizumab and rt‐PA, and a slight but significant reduction of 17.02% of the a‐wave amplitude (p = 0.02; fig. 2b) was detected during the exposure. At washout, the a‐wave amplitude recovered and no significant difference between the a‐wave amplitudes before the application and at the end of the washout was observed (p = 0.07; fig. 2b).
In this analogous manner, we also tested the effects of 20 μg/ml, 60 μg/ml and 200 μg/ml rt‐PA on the a‐wave amplitude. At a concentration of 20 μg/ml rt‐PA, a specific reduction of the a‐wave was not detected during the exposure (p = 0.23; fig. 3b). However, the higher tested concentration of 60 μg/ml induced a significant reduction of the a‐wave amplitude of 18.2% (p<0.01; fig. 3d). However, for both concentrations of rt‐PA, a difference between the a‐wave amplitudes before application and at the end of the washout was not detected, reaching the mean value of a‐wave amplitudes before the application of rt‐PA (p = 0.18 and p = 0.23, respectively; fig. 3b and d).
The application of 200 μg/ml rt‐PA induced a significant reduction of the a‐wave amplitude of 36.6% (p<0.01). At washout, the recovery of the a‐wave amplitude remained incomplete and did not reach the mean value of a‐wave amplitude before the application of rt‐PA (p = 0.01; fig. 3f).
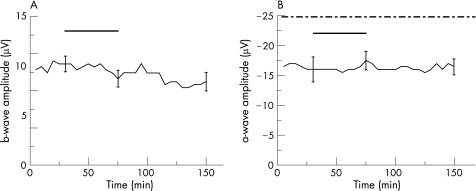
To determine the effects of the vehicle of rt‐PA on retinal function, the dilution of rt‐PA at a concentration of 20 μg/ml rt‐PA was added to the nutrient solution and used as a control. Apart from this variation, the test series were performed as described before. During a 45‐min incubation period, the excipient caused no reduction of the a‐ and b‐wave amplitudes (p = 0.16 and p = 0.40, respectively; fig. 4a and b) and the recordings remained stable throughout the washout (p = 0.40 and p = 0.74, respectively; fig. 4a and b).
Figure 4 Effects of the vehicle on the a‐ and b‐wave amplitude of the isolated perfused bovine retina. The average of representative drug series (both test series, n = 5). After reaching equilibrium at stable ERG amplitudes, the vehicle without the substance rt‐PA was added to the nutrient solution. The black horizontal bar labels the time of the vehicle application. The dash‐dotted line in B marks the time of the additional aspartate application (1 mM). After 45 min, the exposure with the vehicle was discontinued and the perfusion with the nutrient solution resumed. The ERG amplitudes remained stable throughout the washout. Three representative standard deviations are given.
The sheep anti‐bovine IgG was tested on the b‐wave amplitude and used as a negative control for the test series bevacizumab in co‐application with rt‐PA. During exposure with the sheep anti‐bovine IgG, the b‐wave amplitudes remained stable. An alteration of the b‐wave was also not detected during the washout (fig. 5).
Figure 5 Effects of the sheep anti‐bovine IgG on the b‐wave amplitude of the isolated perfused bovine retina. The black horizontal bar marks the time of the application of 0.25 mg/ml sheep anti‐bovine IgG according to the tested concentration of bevacizumab (n = 1). After reaching stable ERG amplitudes, the sheep anti‐bovine IgG was added to the nutrient solution. After 45 min, the exposure was discontinued and the perfusion with the nutrient solution was resumed. The ERG amplitudes remained stable throughout the washout.
Discussion
The condition SRH is associated with a poor visual outcome due to a barrier effect that prevents metabolic exchange between the retina and choriocapillaris, the toxicity of iron released by the haemoglobin and shearing of the outer segments of the photoreceptors.19,20 The prognosis of submacular haemorrhage is further worsened if associated with AMD.2,3,4,5 The short‐term effects of displacing the haemorrhage with rt‐PA seem beneficial, but progressive visual loss related to the underlying CNV negate this benefit, whereby the majority of the eyes lose visual acuity.7 Consequently, co‐application with bevacizumab is suggested, thereby avoiding progression of the underlying disease. The effectiveness of bevacizumab alone in the treatment of neovascular AMD has been previously shown in many non‐controlled clinical studies without detecting retinal toxicity.10,11,21,22,23,24 Due to the intriguing clinical results, a combined intraocular application of bevacizumab and rt‐PA seems to be a promising treatment for SRH caused by neovascular AMD.
However, the toxicity of rt‐PA has previously been studied in the rabbit and cat.25,26 It has been established that evidence of retinal toxicity occurred with intravitreal doses greater than or equal to 50 μg, which corresponds to an intravitreal concentration of 10 μg/ml assuming a vitreous volume of approximately 5 ml. A clinical study showed exudative inferior retinal detachments in all patients who received a dose of 100 μg intravitreally, but in none of the patients who received 50 μg.27 Fundus pigmentary changes were observed with an intravitreal dose of 33 μg, which corresponds to a concentration of 6.6 μg/ml.28 To avoid toxic effects, a maximum intravitreal dose of 50 μg has been recommended, although in some clinical studies higher doses of rt‐PA were used.29,30
Singh et al. described a subretinal injection of 48 μg/0.4 ml according to a concentration of 120 μg/ml, which was twelve‐fold higher than the recommended intravitreally applied concentration of 10 μg/ml.30 However, no evidence of retinal toxicity secondary to rt‐PA in any of the patients has been observed.30
Recapitulating the observations due to the retinal toxicity of rt‐PA, it must be hypothesised that, for rt‐PA, only a narrow therapeutic range exists. The ideal therapeutic concentration of rt‐PA in the treatment of SRH caused by neovascular AMD has not yet been found. For rt‐PA, it has been established that it effectively lyses subretinal clots when injected subretinally around the clotted blood. Therefore, we assume that a subretinal injected concentration of 20 μg/ml is conducive to the thrombolytic characteristics of rt‐PA and to reducing retinal toxicity.
Our results confirmed this consideration and no noteworthy retinal toxicity was detected for the simultaneous application of 20 μg/ml rt‐PA and 0.25 mg/ml bevacizumab, although a small reduction of the a‐wave amplitude was observed during exposure. Considering the negative controls, the small decrease of the a‐wave has to be attributed to the applied active agents. However, a full recovery was detected during the washout.
Therefore, according to our results, a subretinal injection of 20 μg/ml can be combined with an application of 0.25 mg/ml bevacizumab within the surgical displacement of a SRH caused by neovascular AMD. Concentrations of rt‐PA that are higher than 20 μg/ml should not be applied, because a reversible but significant reduction of the a‐wave amplitudes was detected at 60 μg/ml rt‐PA. The highest tested concentration of 200 μg/ml rt‐PA provoked an irreversible reduction of the ERG amplitudes.
The retinal toxicity of rt‐PA is thought to be due to the l‐arginine component of the vehicle. Further evidence for this hypothesis comes from a study of intravitreal injections of aztreonam that also contain l‐arginine in the vehicle, which showed almost equivalent retinal damage when a vehicle equivalent to the aztreonam dose was injected.31,32 However, in our control arm, no evidence for toxic effects on the function of photoreceptors or the higher neuronal network was detected when testing the concentration of the solvent carrier, which was equivalent to a concentration of 20 μg/ml rt‐PA.
The technique of the isolated and perfused bovine retina has often been proven to be a reliable and sensitive tool for pharmacological studies and has been optimized during recent years.13,14 The concentrations of drugs, which are added to the nutrient solution, are precisely specified. The drug effects can be studied separately from systemic effects.
Strong similarities were detected in the drug‐induced changes of the ERG when retinas from human and vertebrate animals were compared.15,16 However, our findings might be limited due to the potentially lower binding properties of bevacizumab to bovine VEGF compared to human VEGF. Nevertheless, the homology of bovine VEGF to human VEGF is about 98%. Therefore, we assume that the results from our animal experimentation with the isolated perfused retina may also be extrapolated to the human retina, according to other safety studies that were published recently.22,33,34
The aim of our study was to evaluate a safe concentration of bevacizumab and rt‐PA for combined treatment. Our study revealed no retinal toxicity for the combined application of bevacizumab and rt‐PA at the tested concentration. The isolated superfused retina is well defined to evaluate the short‐term effects on retinal function. Long‐term effects cannot be excluded with our experimental approach.
We are aware that no definitive conclusions can be drawn based on a single in vitro study. However, we assume that a combined treatment of SRH caused by exudative AMD with bevacizumab and rt‐PA at the tested concentration seems reasonable.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascularization
ERG - electroretinogram
rt‐PA - recombinant tissue plasminogen activator
VEGF - vascular endothelial growth factor
Appendix
We thank Prof Dr Klaus Dietz, Department of Medical Biometry, Eberhard‐Karls‐University Tübingen, for the statistical evaluation.
The Tuebingen Bevacizumab Study Group
Sabine Aisenbrey
Karl Ulrich Bartz‐Schmidt
Christioph Deuter
Faik Gelisken
Salvatore Grisanti
Sylvie Julien
Sigrid Henke‐Fahle
Peter Heiduschka
Werner Inhoffen
Martin Leitritz
Matthias Lüke
Swaantje Peters
Katrin Petermeier
Ulrich Schraermeyer
Ana Sierra
Heike Strotmann
Martin Spitzer
Peter Szurman
Olcay Tatar
Aysegül Tuara
Michael Völcker
Max Warga
Efdal Yörük
Barbara Wallenfels‐Thilo
Focke Ziemssen
Footnotes
Competing interests: None declared.
References
- 1.Klein R, Klein B E, Linton K L. Prevalence of age‐related maculopathy. The Beaver Dam Eye Study. Ophthalmology 199299933–943. [DOI] [PubMed] [Google Scholar]
- 2.Bressler N, Bressler S, Fine S. Age related macular degeneration. Surv Ophthalmol 198832375–413. [DOI] [PubMed] [Google Scholar]
- 3.Scupola A, Coscas G, Soubrane G.et al Natural history of macular subretinal hemorrhage in age‐related macular degeneration. Ophthalmologica 199921397–102. [DOI] [PubMed] [Google Scholar]
- 4.Berrocal M H, Lewis M L, Flynn H W., Jr Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol 1996122486–493. [DOI] [PubMed] [Google Scholar]
- 5.Avery R I, Fekrat S, Hawkins B S.et al Natural history of subfoveal subretinal hemorrhage in age‐related macular degeneration. Retina 199616183–189. [DOI] [PubMed] [Google Scholar]
- 6.Haupert C L, McCuen BW I I, Jaffe G J.et al Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid‐gas exchange for displacement of thick submacular hemorrhage in age‐related macular degeneration. Am J Ophthalmol 2001131208–215. [DOI] [PubMed] [Google Scholar]
- 7.Oshima Y, Ohji M, Tano Y. Pars plana vitrectomy with peripheral retinotomy following preoperative intravitreal tissue plasminogen activator: a modified procedure to drain massive subretinal hemorrhage. Br J Ophthalmol Published Online first: 17 Aug 2006. doi: 10, 1136/ bjo. 2006. 101444. [DOI] [PMC free article] [PubMed]
- 8.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 200425581–611. [DOI] [PubMed] [Google Scholar]
- 9.Kvanta A, Algvere P V, Berglin L.et al Subfoveal fibrovascular membranes in age‐related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 1996371929–1934. [PubMed] [Google Scholar]
- 10.Rosenfeld P J, Mosfeghi A A, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmic Surg Lasers Imaging 200536331–335. [PubMed] [Google Scholar]
- 11.Michels S, Rosenfeld J R, Puliafito C A.et al Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration. Twelve‐week results of an uncontrolled open‐label clinical study. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Fei D, Vanderlaan M.et al Biological activity of bevacizumab, a humanized anti‐VEGF antibody in‐vitro. Angiogenensis 20057335–345. [DOI] [PubMed] [Google Scholar]
- 13.Lüke M. Weiergräber M, Brand C, et al. The isolated perfused bovine retina – A sensitive tool for pharmacological research on retinal function. Brain Res Brain Res Protoc 20051627–36. [DOI] [PubMed] [Google Scholar]
- 14.Sickel W. Respiratory and electrical responses to light stimulation in the retina of the frog. Science 1965148648–651. [DOI] [PubMed] [Google Scholar]
- 15.Lüke C, Walter P, Bartz‐Schmidt K U.et al Effects of antiviral agents on retinal function in vertebrate retina. In: Green K, ed. Advances in ocular toxicology. New York: Plenum Press, 1997107–112.
- 16.Walter P, Lüke C, Sickel W.et al Antibiotics and light responses in superfused bovine retina. Cell Moll Neurobiol 19991987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanawa I, Tateishi T. The effect of aspartate on the electroretinogram of the vertebrate retina. Experientia 1970261311–1312. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld J R, Fung A E, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central vein occlusion. Ophthalmic Surg Lasers Imaging 200636336–339. [PubMed] [Google Scholar]
- 19.Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol 198294762–773. [DOI] [PubMed] [Google Scholar]
- 20.Toth C A, Morse L S, Hjelmeland L M.et al Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol 1991109723–729. [DOI] [PubMed] [Google Scholar]
- 21.Nguyori Q D, Shah S, Tatlipinar S.et al Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 2005891368–1370. [PMC free article] [PubMed] [Google Scholar]
- 22.Manzano R P A, Peyman G A, Khan P.et al Testing intravitreal toxicity of bevacizumab (Avastin). Retina 200626257–261. [DOI] [PubMed] [Google Scholar]
- 23.Maturi R K, Bleau L A, Wilson D L. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina 200626270–274. [DOI] [PubMed] [Google Scholar]
- 24.Lüke M, Warga M, Ziemssen F.et al Effects of bevacizumab on retinal function in isolated vertebrate retina. Br J Ophthalmol. 2006 Sep 901178–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson M W, Olsen K R, Hernandez E.et al Retinal toxicity of recombinant tissue plasminogen activator in the rabbit. Ophthalmology 1990108259–263. [DOI] [PubMed] [Google Scholar]
- 26.Hrach C J, Johnson M W, Hassan A S.et al Retinal toxicity of commercial intravitreal tissue plasminogen activator solution in cat eyes. Arch Ophthalmol 2000118659–663. [DOI] [PubMed] [Google Scholar]
- 27.Hesse L, Schmidt J, Kroll P. Management of acute submacular hemorrhage using recombinant tissue plasminogen activator and gas. Graefes Arch Clin Exp Ophthalmol 1999237273–277. [DOI] [PubMed] [Google Scholar]
- 28.LaPuente C R, Peyman G A.et al Guidelines for intravitreal treatment of vascular occlusions with rtPA (abstract). Invest Ophthalmol Vis Sci 199839(suppl)988 [Google Scholar]
- 29.Hassan A S, Johnson M W, Schneiderman T E.et al Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology 19991061900–1906. [DOI] [PubMed] [Google Scholar]
- 30.Singh R P, Patel C, Sears J E. Management of subretinal macular haemorrhage by direct administration of tissue plasminogen activator. Br J Ophthalmol 200690429–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loewenstein A, Zemel E, Lazar M.et al Drug‐induced retinal toxicity in albino rabbits: the effects of imipenem and aztreonam. Invest Ophthalmol Vis Sci 1993343466–3476. [PubMed] [Google Scholar]
- 32.Rowley S A, Vijayasekaran S, Yu P K.et al Retinal toxicity of intravitreal tenecteplase in the rabbit. Br J Ophthalmol 200488573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feiner L, Barr E E, Shui Y B.et al Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina 200626882–888. [DOI] [PubMed] [Google Scholar]
- 34.Bakri S J, Cameron J D, McCannel C A.et al Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol 2006142162–164. [DOI] [PubMed] [Google Scholar]