Abstract
Objective
To report the functional and morphological outcome of surgical treatment of peripapillary choroidal neovascularisation due to age‐related macular degeneration.
Methods
Consecutive interventional case series of eight patients with extensive peripapillary choroidal neovascularisation and accompanying haemorrhage who underwent subretinal surgery including extraction of the neovascular complex. Ophthalmic examination, including visual acuity testing, colour photography and fluorescein angiography, was performed at baseline and at 3, 6, 9 and 12 months, and then yearly.
Results
Mean follow‐up was 26 months (12–60 months). Preoperative best corrected visual acuity (BCVA) ranged from logMAR (logarithm of minimum angle of acuity) 1.0 (20/200) to logMAR 0.0 (20/20), with a mean of logMAR 0.5 (20/63). Mean postoperative BCVA was logMAR 0.3 (20/40). BCVA improved in six patients, was stable in one patient and deteriorated in one patient. Two years after surgery, one patient developed recurrence of the CNV that was removed surgically. One patient showed retinal detachment 5 years after subretinal surgery.
Conclusions
In this small case series of PPCNV, functional improvement was achieved after surgery in the majority of patients. Surgical extraction of the CNV represents an alternative treatment option in eyes with vision‐threatening extensive PPCNV. Randomised controlled studies seem to be justified to evaluate further the beneficial effect and long‐term functional outcome of this therapy approach.
Peripapillary choroidal neovascularisation (PPCNV) represents a relatively uncommon entity; associations with optic disc drusen, myopia, angioid streaks, ocular histoplasmosis syndrome and uveitis have been reported in younger patients.1,2 In elderly patients, PPCNV can be interpreted as a complication of age‐related macular degeneration (AMD).3 Among all conditions leading to PPCNV, Browning and Frazer2 found a predominance of PPCNV due to AMD. Symptomatic PPCNVs are often large neovascular complexes that may lead to severe visual loss, usually without spontaneous recovery or improvement once subretinal fluid, haemorrhage or extension into the fovea occurs.1,3,4,5 Current treatment options including laser therapy,6,7,8,9 photodynamic therapy (PDT),10 intravitreal injection of antiangiogenic agents, including pegaptanib, ranibizumab and bevacizumab, as well as surgical removal of choroidal neovascularisations (CNVs) are controversially discussed.11,12,13,14,15,16,17 Apart from accompanying haemorrhage impeding visualisation and reducing the effect of treatment, the location of these choroidal lesions represents one of the major problems of laser or photodynamic treatment. The Macular Photocoagulation Study Group (MPSG) has recommended treatment of PPCNV with conventional thermal laser if at least 1.5 clock hours of the retina adjacent to the optic nerve can be spared.6 Furthermore, laser treatment is limited by the high recurrence rate.6,7 The indications for treatment of extrafoveal lesions with PDT have not been defined; according to guidelines, treatment should not extend closer than 200 μm to the edge of the optic nerve. A concern of all treatment options is damage to the papillo‐macular bundle and/or macular pigment epithelium leading to corresponding visual field defects. The surgical approach aims to lower this risk, reduce the recurrence rate and possibly improve vision.
We report here functional and morphological results after submacular surgery in a consecutive interventional case series of eight patients with PPCNV due to AMD in order to determine whether the potential benefits justified further evaluation of this treatment option.
Methods
Eight patients older than 50 years with extensive PPCNV accompanied by subretinal fluid, exudates, haemorrhage and/or retinal pigment epithelium (RPE) detachment were consecutively included in this one‐surgeon interventional case series. In all patients, recent progression of the disease in the affected eye had to be present, with progression defined as (1) decrease of vision, (2) new onset of visual symptoms and/or (3) documented growth of the CNV within the previous 12 weeks. History of previous laser treatment for PPCNV served as the exclusion criterion. In none of the patients was an RPE rip detected. Patients were informed of the experimental nature of the treatment approach, and informed consent was obtained from all patients. The surgical procedure included pars plana vitrectomy, and surgical extraction of the CNV through a small retinotomy at the edge of the CNV. Retinotomy is created at the peripheral edge of the CNV complex parallel to the nerve fibre course aiming to minimise nerve fibre damage. A gas tamponade with a long‐lasting intraocular gas (C3F8) was used in all procedures. In two patients with progressed cataract, vitrectomy was combined with cataract extraction and intraocular lens implantation.
Best corrected visual acuity (BCVA) was determined using a standard Early Treatment Diabetic Retinopathy Study (ETDRS) assessment procedure. At baseline and each visit, patients underwent BCVA testing, ophthalmoscopic examination, visual field testing (static perimetry), fundus photography and fluorescein angiography. Patients were scheduled for regular follow‐up visits the day after surgery and at 3, 6, 9 and 12 months; thereafter a yearly control visit was recommended.
Main outcome measures included assessment of changes from baseline in BCVA scores, with improvement defined as gain of one or more lines on the ETDRS chart along with evaluation of visual field defects and angiographic lesion characteristics. Data were statistically analysed using paired Student's t test for changes in BCVA, with statistical significance defined as p<0.05.
Results
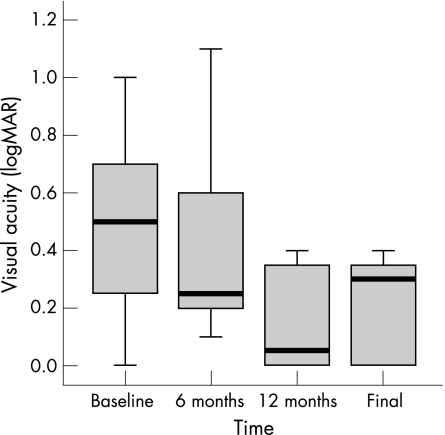
The mean age of patients was 69 years (61–76 years) at the time of surgery. Patient characteristics and BCVA values over time are given in table 1. The CNV was located peripapillary in all eyes without extension of the lesion into the fovea. Extension measured 4–6 clock hours (median 4.5 clock hours). CNV characteristics are given in table 2. Pre‐ and postoperative angiograms of a representative patient are shown in figure 2. Preoperative BCVA ranged from logarithm of minimum angle of acuity (logMAR) 1.0 (20/200) to logMAR 0.0 (20/20), with a mean and median of 0.5 (20/63). Mean postoperative BCVA at 12 months was logMAR 0.26 (median logMAR 0.25 (20/32)) and logMAR 0.3 (median logMAR 0.3 (20/40)) at final examination. BCVA improved in six patients, was stable in one patient and deteriorated in one patient (table 3). Mean improvement of BCVA was 2.8 lines at 12 months and 2.0 lines at final examination; change of BCVA at 12 months from baseline proved statistically significant (p = 0.040); the course of BCVA over time is shown in fig 1.
Table 1 Patient characteristics and course of visual acuity (Snellen) over time.
| Patient | Sex | Age | BCVA | Follow‐up (months) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Final | ||||
| 1 | F | 76 | 20/80 | 20/40 | 20/32 | 20/25 | 20/40 | 60 |
| 2 | F | 62 | 20/50 | 20/80 | 20/80 | 20/40 | 20/40 | 40 |
| 3 | M | 65 | 20/20 | 20/40 | 20/32 | 20/20 | 20/20 | 31 |
| 4 | M | 69 | 20/40 | 20/50 | 20/80 | 20/25 | 20/20 | 22 |
| 5 | M | 72 | 20/32 | 20/25 | 20/25 | 20/20 | 20/20 | 15 |
| 6 | F | 71 | 20/100 | 20/200 | 20/250 | 20/160 | 20/200 | 12 |
| 7 | M | 61 | 20/100 | 20/25 | 20/32 | 20/40 | 20/40 | 12 |
| 8 | F | 76 | 20/200 | 20/40 | 20/40 | 20/50 | 20/50 | 12 |
BCVA, best corrected visual acuity; M, male; F, female.
Table 2 CNV characteristics at baseline and at 12 months.
| Patient | Eye | Extent of CNV (clock hours) | Location of CNV* | Angiographic charasterictics of CNV |
|---|---|---|---|---|
| 1 | OD | 4 | Superior | Haemorrhage |
| 2 | OS | 5 | Nasal/inferior | Haemorrhage |
| 3 | OS | 5 | Superior | Haemorrhage, exudate, fluid, PED |
| 4 | OS | 4 | Inferior/nasal | Haemorrhage |
| 5 | OS | 6 | Nasal/superior | Haemorrhage, exudate, fluid, PED |
| 6 | OS | 5 | Nasal | Hamorrhage, exudate, fibrosis, PED |
| 7 | OD | 4 | Superior | Haemorrhage |
| 8 | OS | 4 | Superior/nasal | Exudate, fluid, PED |
*Location relative to optic disc.
CNV, choroidal neovascularisation; OD, right eye; OS, left eye; PED, pigment epithelium detachment.
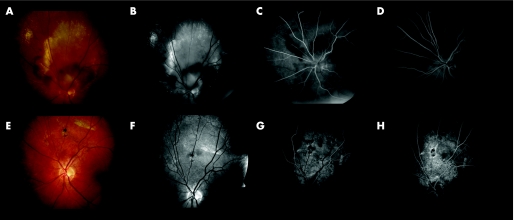
Figure 2 (A) Fundus photograph; (B) red‐free photograph; (C, D) fluorescein angiography (early and late phase) of the right eye of a 65‐year‐old male patient presenting PPCNV. (E–H) At 12 months postoperatively. BCVA remained 20/20.
Table 3 Frequency distribution of change in visual acuity from baseline.
| Change in visual acuity | Patients n (%) |
|---|---|
| ⩾6‐line increase | 1 (12.5) |
| ⩾3‐line increase to <6‐line increase | 3 (37.5) |
| ⩾1‐line increase to <3‐line increase | 2 (25.0) |
| No change | 1 (12.5) |
| ⩾1‐line decrease to <3‐line decrease | 0 (0) |
| ⩾3‐line decrease to <6‐line decrease | 1 (12.5) |
| ⩾6‐line decrease | 0 (0) |
| Total | 8 (100) |
Figure 1 Box blot illustrating change in BCVA over time.
None of the patients presented with significant surgically induced visual field loss or elevated intraocular pressure. All phakic patients showed progression of cataract in the operated eye; in four of six patients, cataract surgery was performed 6–13 months after initial surgery and in two patients cataract surgery has not been performed during the follow‐up period. One patient developed peripheral retinal detachment 5 years after subretinal surgery and 4 years after cataract extraction without affecting the final BCVA. No proliferative vitreoretinopathy was seen in any patient. In one patient, recurrence of the CNV was detected 2 years after surgery at the edge of the excision bed; the CNV was removed surgically and no further recurrence has occurred. In one patient with a history of cardiovascular disease, branch retinal vein occlusion (BRVO) was observed 3 months after surgery during a routine follow‐up visit; her hypertension treatment regimen was improved. This patient was the only patient in our series without improvement of BCVA; interestingly, she rapidly developed symptomatic PPCNV on her fellow eye 1 year after surgery.
Discussion
PPCNV is a relatively uncommon presentation in AMD that can cause significant visual loss without treatment. Natural history of untreated PPCNV reported by the MPSG shows that nearly 25% of eyes had BCVA of 20/500 or worse at 3 years of follow‐up. The MPSG compared visual outcome of laser photocoagulation versus observation in patients with PPCNV associated with presumed ocular histoplasmosis syndrome and idiopathic causes. Treated eyes had only slightly better visual outcome than untreated eyes: 50% of treated eyes compared with 43% of untreated eyes achieved a visual acuity of 20/40 or better.6 The MPSG recommendations for smaller peripapillary lesions are well supported by their data. Laser‐eligible PPCNVs are defined as lesions in which complete treatment would spare at least 1.5 clock hours of the temporal peripapillary area. Yet for larger lesions and those with a large amount of adjacent subretinal haemorrhage, laser treatment is not a primary option. Thermal laser also leads to destruction of the overlying outer retinal layers and occasionally the nerve fibre layer, with the associated visual field defects. In addition to possible injuries of the papillomacular bundle, laser treatment of PPCNV has also been associated with vitreous haemorrhage, optic neuritis and branch arteriole occlusion.18,19,20 In our series, we did not observe visual field defects after surgical excision of the PPCNV. In addition recurrence after treatment of small PPCNVs according to MPSG guidelines appears in a considerable number of patients; these patients are then not eligible for surgical intervention as the CNV complex cannot be excised without major damage to RPE and neurosensory retina.
On the background of our experiences from surgical treatment of subfoveal CNV, we excluded patients with a history of laser treatment in the area of the present CNV as the CNV complex cannot be removed without major damage to the neurosensory retina and the RPE due to strong adhesion of coagulated tissue.
In comparison, PDT lowers the risk of changes of the overlying retina and might be a viable option in the treatment of PPCNV. Rosenblatt10 reported successful photodynamic treatment of PPCNV in a small series of patients. All patients showed improvement or stabilisation of visual acuity and resolution of subfoveal exudation, haemorrhage or fluid. Nerve fibre analysis did not reveal any changes within weeks after treatment. However, current indications approved are subfoveal lesions and require a distance of >200 μm from the edge of the optic nerve. Long‐term data on function and health of the optic nerve after peripapillary PDT are not available. Several concerns have been raised regarding the treatment of tissue close to the optic nerve: first, one might unintentionally also occlude capillaries of the optic nerve when treating an area close to the optic disc; and, secondly, treating an area close to the optic disc implies steady fixation and lack of eye movement during the treatment—a condition unlikely to be achieved in these patients with recent deterioration of central vision. Application of the PDT beam around the optic disc has not been standardised: a variety of different techniques including multiple spots, large spots and the ‘paint brush' technique are currently being investigated.
Pegaptanid sodium, a selective inhibitor of the vascular endothelial growth factor (VEGF) 165 isoform, and ranibizumab, an antibody fragment targeting all known isoforms of VEGF, have recently been approved for treatment of subfoveal CNV due to AMD. The related full‐length antibody bevacizumab is currently approved for intravenous treatment of colo‐rectal cancer; the angiogenic agent is widely used as off‐label application in the treatment of exudative AMD. The safety and efficacy of VEGF inhibitors including pegaptanid and ranibizumab are currently under investigation in prospective, randomised, multicentre, controlled clinical trials including patients with subfoveal lesions exclusively. The first data of these trials investigating pegaptanid and ranibizumab demonstrate encouraging treatment results for this subgroup of AMD patients.21,22 Possible future use of VEGF inhibitors in patients with PPCNV prior to subfoveal extension awaits further determination.
Surgical extraction seems to be a suitable alternative for patients with PPCNV not eligible for laser treatment or PDT, especially large choroidal complexes accompanied by a large amount of haemorrhage, exudation and/or pigment epithelium detachment. In a retrospective review, Bains and colleagues13 found improvement or stabilisation of BCVA in only 6 of 17 patients after surgical removal of PPCNV. In a later retrospective series, Kokame17 reported improved or stable BCVA in 7 of 8 patients suffering from fovea‐sparing PPCNV. Blinder16 published details of successful surgical treatment of PPCNV secondary to AMD; 64% of patients had stable or improved vision postoperatively. Improvement correlated with preoperative BCVA: patients with worse visual function showed major improvement. In our series we achieved improvement of visual function in 7 of 8 cases after surgical excision of the peripapillary neovascular complex. Enthusiasm was limited by the considerable occurrence of complications, including one case of recurrent CNV, one case of retinal detachment and one case of BRVO. Progression of cataract after vitrectomy and gas tamponade in this group of patients was not surprising; given the mean age of the patients, we do not consider this side effect a relevant complication with regard to long‐term development of visual function.
One major problem when comparing treatment results in series including patients with PPCNV secondary to presumed ocular histoplasmosis syndrome and AMD lies in the well‐known difference in architecture of CNV with regard to its location: CNV in presumed ocular histoplasmosis syndrome is located anterior to the RPE, thus enabling surgical extraction without associated RPE cell damage. In AMD, subfoveal CNV is most often located beneath the RPE; thus surgical extraction of the CNV leads to RPE damage, as several histopathological studies of excised CNV specimens have demonstrated. However, anatomical situation in the neighbourhood of the optic nerve is unique; thus, experience from subfoveal lesions cannot easily be translated.
Kies and Bird3 reported a high rate (25%) of similar lesions occurring in the same location in the second eye during a comparable follow‐up period. In our series, one patient developed PPCNV on the fellow eye 1 year after initial presentation. Hence, close inspection of the fellow eyes at regular follow‐up visits is recommended given the considerable risk of eventual bilateral involvement.
The study is limited by representing a single‐centre case series lacking a control group, and the smaller sample size compared with published multicentre clinical trials; these limitations introduce potential for inadvertent bias, such as with patient selection and time of cataract surgery.
The results from our small interventional case series suggest that surgical treatment of extensive PPCNV without foveal involvement has the potential to stabilise or even improve visual acuity and avoid growth of the CNV into the fovea. In comparison with laser photocoagulation, surgical excision does not seem to damage the overlying neurosensory retina or affect the optic nerve, one of our concerns when applying PDT in this area. On the other hand, the risk of ocular and/or general complications related to the surgical intervention in these elderly patients has to be taken into careful consideration. Further evaluation of this treatment approach seems to be justified. Randomised prospective trials comparing surgical extraction of PPCNV with PDT and novel treatment regimens including antiangiogenic therapy would be critical to answer the question of beneficial effect and complication rates of the different approaches—given the small number of affected patients, this aim will be difficult to achieve.
Abbreviations
AMD - age‐related macular degeneration
BCVA - best corrected visual acuity
BRVO - branch retinal vein occlusion
CNV - choroidal neovascularisation
ETDRS - Early Treatment Diabetic Retinopathy Study
logMAR - logarithm of minimum angle of acuity
MPSG - Macular Photocoagulation Study Group
PDT - photodynamic therapy
PPCNV - peripapillary choroidal neovascularisation
RPE - retinal pigment epithelium
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None.
Presented at the 19th Annual Congress of the German Retina Society, Kiel, Germany, June 2006.
References
- 1.Lopez P F, Green W R. Peripapillary subretinal neovascularization. A review. Retina 199212147–171. [PubMed] [Google Scholar]
- 2.Browning D J, Fraser C M. Ocular conditions associated with peripapillary subretinal neovascularization, their relative frequencies, and associated outcomes. Ophthalmology 20051121054–1061. [DOI] [PubMed] [Google Scholar]
- 3.Kies J C, Bird A C. Juxtapapillary choroidal neovascularization in older patients. Am J Ophthalmol 198810511–19. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri G, Archer D B, Johnston P B. Peripapillary subretinal neovascular membranes: the natural history. Eye 19937398–402. [DOI] [PubMed] [Google Scholar]
- 5.Ruben S, Palmer H, Marsh R J. The visual outcome of peripapillary choroidal neovascular membranes. Acta Ophthalmol 199472118–121. [DOI] [PubMed] [Google Scholar]
- 6.Marsh M J, Fine S L, Alexander J.et al Macular Photocoagulation Study Group. Laser photocoagulation for neovascular lesions nasal to the fovea. Results from clinical trials for lesions secondary to ocular histoplasmosis or idiopathic causes. Arch Ophthalmol 199511356–61. [PubMed] [Google Scholar]
- 7.Cialdini A P, Jalkh A E, Trempe C L.et al Argon green laser treatment of peripapillary choroidal neovascular membranes. Ophthalmic Surg 19892093–99. [PubMed] [Google Scholar]
- 8.Turcotte P, Maguire M G, Fine S L. Visual results after laser treatment for peripapillary choroidal neovascular membranes. Retina 199111295–300. [DOI] [PubMed] [Google Scholar]
- 9.Flaxel C J, Bird A C, Hamilton A M.et al Partial laser ablation of massive peripapillary subretinal neovascularization. Ophthalmology 19961031250–1259. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt B J, Shah G K, Blinder K. Photodynamic therapy with verteporfin for peripapillary choroidal neovascularization. Retina 20052533–37. [DOI] [PubMed] [Google Scholar]
- 11.Atebara N H, Thomas M A, Holekamp N M.et al Surgical removal of extensive peripapillary choroidal neovascularization associated with presumed ocular histoplasmosis syndrome. Ophthalmology 19981051598–1605. [DOI] [PubMed] [Google Scholar]
- 12.Uemura A, Thomas M A. Visual outcome after surgical removal of choroidal neovascularization in pediatric patients. Arch Ophthalmol 20001181373–1378. [DOI] [PubMed] [Google Scholar]
- 13.Bains H S, Patel M R, Singh H.et al Surgical treatment of extensive peripapillary choroidal neovascularization in elderly patients. Retina 200323469–474. [DOI] [PubMed] [Google Scholar]
- 14.Kertes P J. Massive peripapillary subretinal neovascularization: an indication for submacular surgery. Retina 200424219–225. [DOI] [PubMed] [Google Scholar]
- 15.Mateo C, Moreno J G, Lechuga M.et al Surgical removal of peripapillary choroidal neovascularization associated with optic nerve drusen. Retina 200424739–745. [DOI] [PubMed] [Google Scholar]
- 16.Blinder K J, Shah G K, Thomas M A.et al Surgical removal of peripapillary choroidal neovascularization associated with age‐related macular degeneration. Ophthalmic Surg Lasers Imaging 200536358–364. [PubMed] [Google Scholar]
- 17.Kokame G T, Yamamoka S. Subretinal surgery for peripapillary subretinal neovascular membranes. Retina 200525564–569. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg M F, Herbst R W. Acute complications of argon laser photocoagulation. Epipapillary and peripapillary neovascularization. Arch Ophthalmol 197389311–318. [DOI] [PubMed] [Google Scholar]
- 19.Swartz M, Apple D J, Creel D. Sudden severe visual loss associated with peripapillary burns during panretinal argon photocoagulation. Br J Ophthalmol 198367517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom S M. Thermal papillitis after dye red photocoagulation of a peripapillary choroidal neovascular membrane. Retina 199010261–264. [DOI] [PubMed] [Google Scholar]
- 21.Gragoudas E S, Adamis A P, Cunningham E T., Jret al Pegaptanib for neovascular age‐related macular degeneration. N Engl J Med 20043512805–2816. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld P J, Brown D M, Heier J S.et al Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med 20063551419–1431. [DOI] [PubMed] [Google Scholar]