Abstract
Objective
Osteopontin (OPN) has been found to be valuable in diagnosis and predicting the prognosis of a variety of malignancies. The aims of the present study are to evaluate the usefulness of plasma OPN level for predicting gastric cancer development, invasion and survival.
Patients and Methods
One hundred and thirty two gastric cancer patients and 93 healthy controls were enrolled. Real‐time quantitative reverse‐transcription polymerase chain reaction and immunohistochemical staining were used to detect OPN expression in gastric cancer tissues. Plasma levels of OPN were measured by enzyme‐linked immunosorbent assay. Plasma OPN levels were compared with gastric cancer development, clinicopathological features and outcomes.
Results
Expression of OPN mRNA was significantly higher in gastric cancer tissues compared with non‐tumour tissues. Most OPN immunoactivity was localised to cancer cells. The median plasma OPN level was significantly higher in patients than in controls (p<0.0001), and significantly higher in patients with advanced stages, serosal invasion, lymph node metastasis, lymphatic invasion, venous invasion and liver metastasis. Logistic regression showed that high plasma OPN level (greater than 67.3 ng/ml) is significantly associated with advanced stages, serosal invasion, lymph node metastasis, lymphatic invasion, venous invasion and liver metastasis. Plasma OPN level demonstrated significant association with patient survival (p<0.0001), especially in the subgroups with invasive phenotypes. On Cox multivariate analysis, elevated plasma OPN level was an independent risk factor for poor survival (p<0.0001).
Conclusions
Elevated plasma OPN level is significantly associated with gastric cancer development, invasive phenotypes and survival. Plasma OPN level may have potential usefulness as a diagnostic and prognostic factor for gastric cancer.
Keywords: osteopontin (OPN), plasma, gastric cancer, invasion, survival
Gastric cancer remains a leading cause of cancer mortality, despite a worldwide decline in incidence. In Asian countries, gastric cancer is one of the most prevalent tumours and the leading cause of cancer death.1 In the Western world, more than 80% of gastric cancer patients have advanced cancer on diagnosis with poor prognosis.2 Complete resection of the tumour and adjacent lymph nodes is the only proven, effective curative treatment.3 Unfortunately, the accuracy of current preoperative staging is limited, particularly for depth of invasion, lymph node involvement and distant metastasis.4 Developing new biomarkers to identify the subgroup of gastric cancer patients with invasive phenotypes will be helpful for avoiding inappropriate attempts at curative surgery.
RNA‐based global gene expression strategies are powerful approaches to identifying new cancer markers. Two studies using these strategies have suggested that osteopontin (OPN) is a potential marker for gastric cancer invasiveness.5,6 Lee et al5 used cDNA microarrays to identify differentially expressed genes in gastric cancer tissues and their surrounding gastric mucosa tissues and found 55‐fold OPN overexpression in gastric cancer tissues. Using a highly metastatic gastric cancer cell line model, Fukui et al. found that OPN expression in liver metastatic variant cells is 2.7–10.2 times higher than in the parent cells.6 In an immunohistochemical staining study, co‐immunoreaction of OPN and its receptor, CD44v9, in gastric cancer tumour cells correlated with the degree of lymphatic vessel invasion and lymph node metastasis.7 Recent studies have consistently reported that OPN mRNA and protein expression in cancer tissues is closely related to invasion and metastasis of gastric cancer.8,9
OPN tissue expression and plasma levels have been reported to be diagnostic tumour markers and correlate well with prognosis for a variety of malignancies.15,16,17,18 However, the application of plasma OPN level as a biomarker for gastric cancer has not been investigated. In the present study, we evaluate the usefulness of plasma OPN level for predicting gastric cancer development, invasion and survival.
Patients and methods
Study subjects
Since January 1998, blood samples have been prospectively collected from individuals participating in two national projects to investigate the risk factors of gastric cancer in Taiwan (the National Taiwan University Hospital cohort). All patient‐derived specimens were collected and archived under protocols approved by the institutional review boards of the parent institutions. A full verbal explanation of the study was given to all participants. They consented to participate on a voluntary basis. Patients with newly diagnosed gastric cancer undergoing gastrectomy in the inpatient unit and outpatient cancer clinics of four major medical centres in Taiwan were enrolled. Inclusion and exclusion criteria were detailed previously.19,20 Gastric adenocarcinoma was histopathologically confirmed by surgical specimens. No patients in this study had been prescribed chemotherapy before surgery, and there was no evidence of any other malignancy. In total, we studied 132 consecutive patients with gastric cancer, for whom complete clinical data and a plasma sample were available. All recruited patients had been followed up for at least 5 years.
Control blood samples were obtained from 93 individuals who visited health examination clinics with minimal gastritis or normal appearance of the gastric mucosa on gastroscopic examination. The controls were matched by age (±3 years) and date of blood collection (±3 months).
To verify the diagnostic and prognostic values of plasma OPN level, we used an independent cohort including 39 patients and 50 health controls from Taichung Veterans General Hospital (the VGH validation cohort). The controls were matched by age (±3 years) and date of blood collection (±3 months). We used the same inclusion and exclusion criteria as the first study cohort.
Real‐time quantitative reverse transcription polymerase chain reaction
Detection of OPN mRNA in gastric cancer tissues and surrounding non‐tumour tissues was performed using the method and primer sequences described in the manufacturer's instructions. OPN primers and probes were purchased from ABI (Applied Biosystems, Foster City, California, USA). Total RNA was isolated using Trizol reagent, and 2 μg of RNA from each sample were reverse transcribed using SuperScript II RT (Invitrogen, Carlsbad, California, USA) in total reaction volume of 20 μl. One microlitre of reverse‐transcription product (cDNA) was amplified by polymerase chain reaction (PCR) with Taq DNA polymerase. Expression of TBP for each template was detected in the same PCR run as an internal control for efficiency of reverse transcription and amount of RNA. There were a total of 35 PCR cycles, with each cycle consisting of a denaturation step (95 °C for 1 min), an annealing step (60 °C for 1 min) and an elongation step (72 °C for 1 min).
Immunohistochemical analysis
Tissue sections from gastric cancer samples were deparaffined in xylene and rehydrated in ethanol, then immersed in 3% hydrogen peroxide‐methanol solution for 10 min at room temperature to inhibit endogenous peroxidase activity. Sections were washed twice in phosphate‐buffered saline (PBS) buffer for 5 min, and placed in 10% non‐immune serum (goat) for 10 min. Sections were then incubated with mouse anti‐OPN monoclonal antibodies (Novocastra Laboratories Ltd, Newcastle upon Tyne, UK) at a dilution of 1:50 overnight at 4 °C, and washed twice in PBS, before being incubated in appropriate biotinylated secondary antibody for 10 min. The sections were washed again in PBS twice for 5 min. Slides were incubated in streptavidin/peroxidase conjugate (Zymed Laboratories, Inc.) for 10 min, before being washed twice in PBS for 5 min. Slides were subsequently incubated in DAB (3‐3′‐diamono‐benzidine) solution for 5 min, then washed in water, counterstained with haematoxylin for 2 s, washed thoroughly in running tap water, dehydrated and mounted.
Measurement of plasma OPN concentration
OPN Quantikine ELISA kits were purchased from R&D Systems Europe (product code DOST00). This assay kit precoats a monoclonal antibody specific for OPN onto a microplate to bind OPN in the samples. The sensitivity limit of the assay was 0.011 ng/ml. Plasma OPN concentrations were measured in samples prepared in tubes coated with lithium heparin. All samples were preserved at −80 °C. Plasma samples were diluted and the immunoassays were performed following the manufacturer's instructions. All assays were duplicated. A subset of samples was reassayed six times in every ELISA plate for quality control. The inter‐assay variability was 4.8%.
Statistical analysis
The relative measures of OPN expression, 2−ΔΔCT, on real‐time RT‐PCR in gastric cancer tissues and surrounding non‐tumour tissues were compared using paired Student's t‐test on log‐transformed values. The demographic characteristics of patients and controls were compared using the χ2‐square test and Student's t‐test. Because of the non‐Gaussian distribution of data for the plasma OPN levels in study subjects, the differences in plasma OPN values were compared using a nonparametric Mann Whitney U test. Analyses of nonparametric receiver‐operating characteristics (ROC) were performed to calculate the cutoff values according to the most accurate value obtained. Logistic regression analyses were conducted to evaluate the associations between plasma OPN levels and clinicopathological features of gastric cancer patients. To determine whether plasma OPN concentration is an independent prognostic factor for survival, hazards ratios were calculated using the Cox proportional hazards model. Data were analysed via the SPSS program for Windows, version 11.0 (SPSS Inc., Chicago, Illinois, USA). Analyses of nonparametric ROCs were performed via the STATA program for Windows, version 8.0 (Stata Corporation, College Station, Texas, USA).
Results
OPN expression in gastric cancer tissues
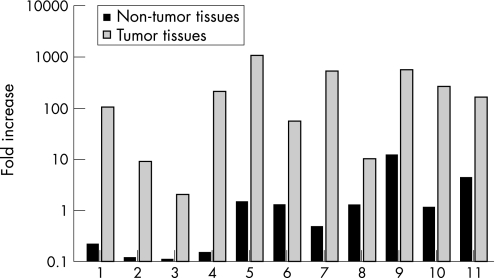
To validate overexpression of OPN in gastric cancer, gastric cancer tissues and surrounding non‐tumour tissues were examined using real‐time RT‐PCR with non‐tumour tissue of sample 10 as the reference. Significantly higher OPN expression was observed in the 11 samples of gastric cancer tissues compared with their surrounding non‐tumour tissues (p<0.0001) (fig 1).
Figure 1 Relative quantitation of osteopontin (OPN) in gastric cancer tissues and neighbouring non‐tumour tissues. Expression differences were obtained for samples of 11 gastric cancer tissues and neighbouring non‐tumour tissues using paired Student's t‐test on log‐transformed values (p<0.0001). Each value is expressed as the mean of duplicate assays.
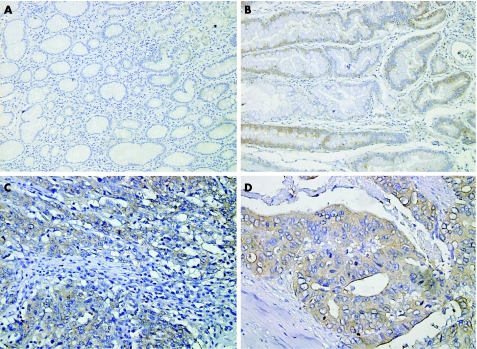
Immunohistochemical staining was performed to localise OPN expression in gastric cancer tissues. OPN immunoreactivity was not observed in the epithelium or stroma of the healthy gastric tissues. In atrophic gastritis, mild OPN immunoreactivity was found in epithelial cells, but not in stromal cells. In gastric cancer tissues, most positive staining was localised to the cancer cell cytoplasm with diffuse staining pattern (fig 2).
Figure 2 Relative quantitation of osteopontin (OPN) expression in gastric cancer tissues. A–D, Immunolocalisation of OPN in human gastric tissues. OPN expression is indicated by brown staining and is immunolocalised using anti‐OPN monoclonal antibody and avidin–biotin peroxidase complex and method with diaminobenzidine as the chromogen. The sections were counterstained with haematoxylin. A, Healthy gastric epithelial cells with no expression of OPN. B, Epithelial cells of atrophic gastritis with weak expression of OPN. C and D, Gastric adenocarcinoma with OPN expression in cancer cells. Magnification: A, B ×250; C ×400; D ×600.
Associations between plasma OPN levels and occurrence of gastric cancer
Plasma OPN levels were analysed in 132 gastric cancer patients and 93 controls. There were no age or gender differences between gastric cancer patients and controls. The median plasma concentration of OPN was significantly higher in gastric cancer patients than in control subjects (78.6 vs 55.6 ng/ml, p<0.0001). To further examine the predictive values of plasma OPN levels, cutoff values were calculated on analyses of ROCs, according to the most accurate value obtained, and predictive probability was determined. Gastric cancer occurrence, serosal invasion, lymph node metastasis and liver metastasis could be predicted with accuracies of 63.6%, 62.9%, 65.2% and 83.3%, respectively (table 1).
Table 1 Cutoff values of plasma osteopontin levels and their sensitivity, specificity and accuracy for predicting gastric cancer occurrence, serosal invasion, lymph node metastasis and liver metastasis.
| Cutoff value (ng/ml) | Predictive probability (%) | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | ||
| All subjects | ||||
| Occurrence | 67.3 | 58.3 | 69.9 | 63.6 |
| Serosal invasion | 78.6 | 61.3 | 65.4 | 62.9 |
| Lymph node metastasis | 73.0 | 65.1 | 65.3 | 65.2 |
| Liver metastasis | 111.2 | 87.5 | 83.1 | 83.3 |
| Helicobacter pylori seropositive subjects | ||||
| Occurrence | 67.3 | 58.6 | 77.8 | 65.2 |
| Serosal invasion | 80.0 | 60.4 | 66.7 | 63.0 |
| Lymph node metastasis | 80.7 | 60.9 | 71.4 | 65.4 |
| Liver metastasis | 129.8 | 80.0 | 88.2 | 87.7 |
In subjects who were Helicobacter pylori seropositive, the accuracies of plasma OPN to predict gastric cancer occurrence, serosal invasion, lymph node metastasis and liver metastasis were 65.2%, 63.0%, 65.4% and 87.7% respectively. Plasma OPN did not have significantly better diagnostic value in subjects who were H pylori seropositivity (table 1). We also evaluated the potential interaction between H pylori serology and plasma OPN level. We found the medians of plasma OPN levels between patients with gastric cancer who were H pylori seropositive and H pylori seronegative were not significantly different (table 2).
Table 2 Plasma median osteopontin levels in patients with gastric cancer with different clinicopathological features.
| Plasma OPN level (ng/ml) Median (mean±SE) | p Value* | |
|---|---|---|
| Helicobacter pylori | ||
| Seronegative (n = 51) | 78.6 (94.2±10.9) | |
| Seropositive (n = 81) | 78.0 (86.2±7.2) | NS |
| Stage | ||
| I (n = 42) | 59.3 (66.1±8.2) | |
| II (n = 23) | 63.7 (74.5±8.2) | NS† |
| III (n = 40) | 89.9 (96.9±10.6) | 0.003† |
| IV (n = 27) | 97.0 (126.9±18.8) | <0.001† |
| Serosal invasion | ||
| Absent (n = 52) | 66.3 (73.6±7.4) | |
| Present (n = 80) | 84.2 (99.5±8.7) | 0.017 |
| Lymph node metastasis | ||
| Absent (n = 49) | 63.3 (68.9±7.1) | |
| Present (n = 83) | 90.2 (101.6±8.5) | 0.003‡ |
| pN1 (n = 56) | 82.9 (94.7±9.4) | 0.018‡ |
| pN2, N3 (n = 27) | 92.4 (115.8±17.3) | 0.003‡ |
| Lymphatic invasion | ||
| Absent (n = 76) | 64.5 (76.0±6.9) | |
| Present (n = 56) | 91.8 (107.5±10.5) | 0.003 |
| Venous invasion | ||
| Absent (n = 77) | 63.7 (82.8±8.3) | |
| Present (n = 55) | 84.1 (98.5±8.9) | 0.035 |
| Liver metastasis | ||
| Absent (n = 124) | 75.6 (82.2±5.3) | |
| Present (n = 8) | 166.5 (200.2±45.3) | 0.002 |
*p Value calculated by non‐parametric Mann Whitney U test.
†Compared with stage I.
‡Compared with no lymph node metastasis.
NS, non‐significant; SE, standard error.
Associations between plasma OPN levels and clinicopathological characteristics of gastric cancer
We then compared plasma OPN levels among gastric cancer patients with different clinicopathological features. Median plasma OPN concentrations were significantly higher in patients with advanced stages (stage III, p = 0.003; stage IV, p<0.001), serosal invasion (p = 0.017), lymph node metastasis (p = 0.003), lymphatic invasion (p = 0.003), venous invasion (p = 0.035), and liver metastasis (p = 0.002) (table 2). Among the patients with lymph node metastasis, those with pN2 or pN3 stages had a higher median plasma OPN levels than those with pN1 stage (table 2).
To examine the predictive value of plasma OPN for different clinicopathological characteristics, we conducted logistic regression analyses. We defined elevated plasma OPN levels according to the best predictive values calculated on ROC analyses for serosal invasion (>78.6 ng/ml), lymph node metastasis (>73.0 ng/ml), liver metastasis (>111.2 ng/ml) and used the criteria >67.3 ng/ml for analyses of other parameters. Elevated plasma OPN level was associated with age (p = 0.003), advanced stages (stage III: p = 0.004; stage IV: p = 0.002), serosal invasion (p = 0.003), lymph node metastasis (p = 0.001), lymphatic invasion (p = 0.004), venous invasion (p<0.001), and liver metastasis (p = 0.005) (table 3).
Table 3 Association between plasma osteopontin levels and clinicopathological features of gastric cancer.
| Plasma OPN levels | p Value | |||
|---|---|---|---|---|
| Normal | Elevated | Odds ratio (95% CI)* | ||
| Helicobacter pylori | (⩽67.3 ng/ml) | (>67.3 ng/ml) | ||
| Seronegative | 20 (35.7%) | 31 (40.8%) | 1 | |
| Seropositive | 36 (64.3%) | 45 (59.2%) | 0.81 (0.40 to 1.65) | NS |
| Age | (⩽67.3 ng/ml) | (>67.3 ng/ml) | ||
| ⩽60 years | 29 (51.8%) | 20 (26.3%) | 1 | |
| >60 years | 27 (48.2%) | 56 (73.7%) | 3.01 (1.45 to 6.25) | 0.003 |
| Sex | (⩽67.3 ng/ml) | (>67.3 ng/ml) | ||
| Female | 23 (41.1%) | 23 (30.3%) | 1 | |
| Male | 33 (58.9%) | 53 (69.7%) | 1.61 (0.78 to 3.31) | NS |
| Stage | (⩽67.3 ng/ml) | (>67.3 ng/ml) | ||
| I | 26 (46.4%) | 16 (21.1%) | 1 | |
| II | 12 (21.4%) | 11 (14.5%) | 1.49 (0.53 to 4.17) | NS† |
| III | 12 (21.4%) | 28 (36.8%) | 3.79 (1.51 to 9.51) | 0.004† |
| IV | 6 (10.7%) | 21 (27.6%) | 5.69 (1.89 to 17.09) | 0.002† |
| Serosal invasion | (⩽78.6 ng/ml) | (>78.6 ng/ml) | ||
| Absent | 34 (52.3%) | 18 (26.9%) | 1 | |
| Present | 31 (47.7%) | 49 (73.1%) | 3.21 (1.49 to 6.92)* | 0.003 |
| Lymph node metastasis | (⩽73 ng/ml) | (>73.0 ng/ml) | ||
| Absent | 32 (51.6%) | 17 (24.3%) | 1 | |
| Present | 30 (48.4%) | 53 (75.7%) | 3.88 (1.75 to 8.61)* | 0.001 |
| Lymphatic invasion | (⩽67.3 ng/ml) | (>67.3 ng/ml) | ||
| Absent | 40 (71.4%) | 36 (47.4%) | 1 | |
| Present | 16 (28.6%) | 40 (52.6%) | 3.16 (1.45 to 6.91)* | 0.004 |
| Venous invasion | (⩽67.3 ng/ml) | (>67.3 ng/ml) | ||
| Absent | 42 (75.0%) | 35 (46.1%) | 1 | |
| Present | 14 (25.0%) | 41 (53.9%) | 4.24 (1.88 to 9.56)* | 0.001 |
| Liver metastasis | (⩽111.2 ng/ml) | (>111.2 ng/ml) | ||
| Absent | 103 (98.1%) | 21 (77.8%) | 1 | |
| Present | 2 (1.9%) | 6 (22.2%) | 11.09 (2.04 to 60.29)* | 0.005 |
*Odds ratio adjusted for age and sex.
†Compared with stage I.
95% CI, 95% confidence interval; NS, non‐significant.
Associations between plasma OPN levels and survival
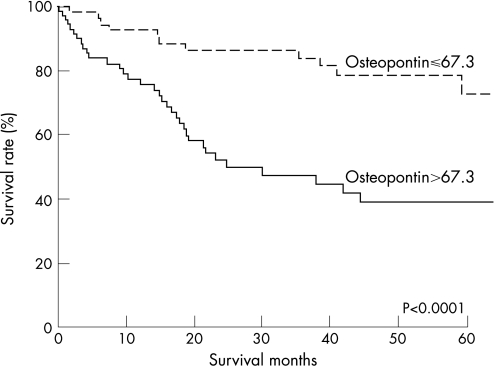
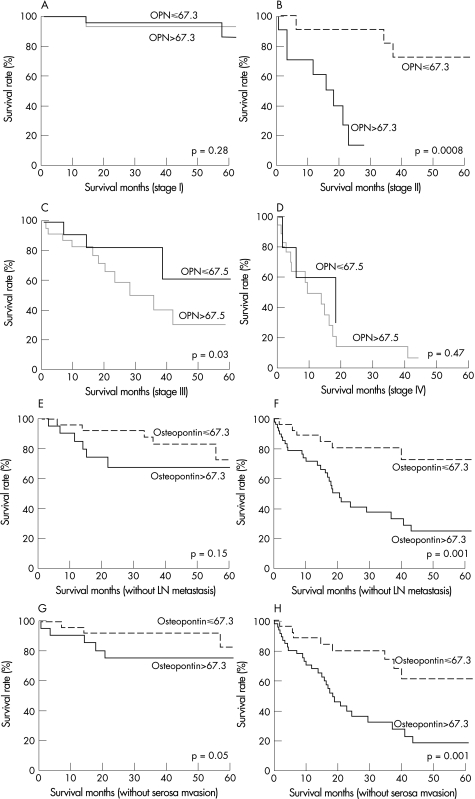
Kaplan–Meier estimates of survival for patients with different plasma OPN levels are shown in Figure 3. The survival rates for patients with elevated plasma OPN level (>67.3 ng/ml) were significantly lower than the survival rates of those with normal plasma OPN level (P<0.0001). Subgroup analyses according to stages, lymph node metastasis and serosal invasion status are shown in fig 4. Elevated plasma OPN level was associated with poor survival in gastric cancer patients with stage II disease (p = 0.0008) and stage III disease (p = 0.03), patients with lymph node metastasis (p = 0.001), patients without serosal invasion (p = 0.05) and patients with serosal invasion (p = 0.001). Patients with elevated plasma OPN level had significantly poorer prognosis, especially in the subgroups with invasive phenotypes, such as those with advanced stage, lymph node metastasis and serosal invasion.
Figure 3 Kaplan–Meier estimates of survival for patients with different plasma OPN levels. The survival rates for patients with plasma OPN>67.3 ng/ml were significantly lower than for patients with plasma OPN⩽67.3 ng/ml (p<0.0001 by the log‐rank test).
Figure 4 Kaplan–Meier curves for overall survival according to different plasma OPN levels. Stage I gastric cancer (panel A), stage II gastric cancer (panel B), stage III gastric cancer (panel C), stage IV gastric cancer (panel D), without lymph node metastasis (panel E), with lymph node metastasis (panel F), without serosal invasion (panel G) and with serosal invasion (panel H). Patients with elevated plasma OPN (>67.3 ng/ml) had significantly worse prognosis, especially in the stage II, stage III, lymph node metastasis and serosal invasion subgroups.
On Cox univariate proportional hazards analysis, we found that patients with stage III or stage IV (p<0.001), serosal invasion (p<0.001), lymphatic invasion (p = 0.002), venous invasion (p<0.001), lymph node metastasis (p = 0.002), liver metastasis (p<0.001) and plasma OPN levels (p<0.001) were of prognostic significance for poor overall survival (table 4). On multivariate analysis, only serosal invasion (p = 0.001), liver metastasis (p = 0.009) and high plasma OPN level (p = 0.0001) were independent risk factors for poor overall survival (table 5).
Table 4 Univariate analysis for the predictors of mortality in gastric cancer patients.
| Hazards ratio | (95% CI) | p Value | |
|---|---|---|---|
| Age | |||
| >60 vs ⩽60 | 1.64 | (0.88 to 3.03) | NS |
| Gender | |||
| Male vs female | 1.40 | (0.72 to 2.71) | NS |
| Stage | |||
| Stage III, IV vs stage I, II | 3.98 | (2.09 to 7.59) | <0.001 |
| Depth of invasion | |||
| Serosal vs non‐serosal invasion | 4.78 | (2.21 to 10.35) | <0.001 |
| Lymph node metastasis | |||
| Yes vs no | 2.89 | (1.46 to 5.74) | 0.002 |
| Lymphatic invasion | |||
| Yes vs no | 2.55 | (1.42 to 4.56) | 0.002 |
| Venous invasion | |||
| Yes vs no | 2.93 | (1.63 to 2.25) | <0.001 |
| Liver metastasis | |||
| Yes vs no | 6.02 | (2.30 to 15.79) | <0.001 |
| Osteopontin | |||
| >67.3 vs ⩽67.3 | 3.85 | (1.95 to 7.61) | <0.001 |
| Osteopontin | |||
| As a continuous variable | 1.009 | (1.005 to 1.012) | <0.001 |
Table 5 Multivariable Cox proportional hazards model analysis for prediction of mortality in gastric cancer patients.
| Hazards Ratio | 95% CI | p Value | |
|---|---|---|---|
| Serosal invasion | |||
| Yes vs no | 3.97 | (1.81 to 8.73) | 0.001 |
| Liver metastasis | |||
| Yes vs no | 3.67 | (1.38 to 9.79) | 0.009 |
| Osteopontin | |||
| >67.3 vs ⩽67.3 | 3.38 | (1.70 to 6.73) | 0.001 |
Because plasma OPN level is a continuous variable, the use of an arbitrary cut‐off value of 67.3 ng/ml may be misleading. We also analysed plasma OPN level as a continuous variable on the Cox proportional hazard model. On univariable analysis, patients with high plasma OPN levels were of prognostic significance for poor overall survival (p<0.001) (table 5). On multivariate analysis, only serosal invasion (p<0.001) and high plasma OPN level (p<0.001) were independent risk factors for poor overall survival (table 6).
Table 6 Multivariable Cox proportional hazards model analysis for prediction of mortality in gastric cancer patients.
| Hazards ratio | 95% CI | p Value | |
|---|---|---|---|
| Serosal invasion | |||
| Yes vs no | 4.32 | (1.97–9.47) | <0.001 |
| Osteopontin | |||
| As a continuous variable | 1.008 | (1.003–1.012) | <0.001 |
Validation of the diagnostic and prognostic values
To assess the robustness of plasma OPN level as a diagnostic and prognostic factor in gastric cancer, we used an independent set of blood samples (the VGH validation cohort) to validate the results. We found that patients with gastric cancer had significantly higher median plasma OPN level compared with controls (p<0.001). If we used the same cutoff value at 67.3 ng/ml, the sensitivity was 82.1% and specificity was 78.0%.
Median plasma OPN concentrations were significantly higher in patients with stage II (p = 0.048), stage III (<0.001), stage IV (p = 0.003), serosal invasion (p = 0.002), lymph node metastasis (p = 0.002), lymphatic invasion (p<0.001), and venous invasion (p = 0.001) (table 7). Because the VGH validation cohort patients are followed shortly, we do not have sufficient survival data to conduct Cox proportional hazard analysis.
Table 7 Plasma median OPN levels in the VGH validation cohort.
| Plasma OPN level (ng/ml) Median (mean ± SE) | p Value* | |
|---|---|---|
| Gastric cancer patients (n = 39) | 107.0 (110.8±7.8) | |
| Control (n = 50) | 51.7 (49.4±3.4) | <0.001 |
| Stage | ||
| I (n = 6) | 62.0 (55.7±6.7) | |
| II (n = 3) | 75.0 (92.0±20.5) | 0.048† |
| III (n = 20) | 106.0 (109.7±7.8) | <0.001† |
| IV (n = 10) | 143.6 (151.7±17.0) | 0.003† |
| Serosal invasion | ||
| Absent (n = 7) | 63.9 (67.5±13.7) | |
| Present (n = 32) | 116.3 (120.3±8.1) | 0.002 |
| Lymph node metastasis | ||
| Absent (n = 7) | 63.9 (66.9±12.4) | |
| Present (n = 32) | 116.3 (120.4±8.2) | 0.002 |
| Lymphatic invasion | ||
| Absent (n = 14) | 70.5 (75.2±8.5) | |
| Present (n = 25) | 119.9 (130.7±8.9) | <0.001 |
| Venous invasion | ||
| Absent (n = 18) | 79.8 (86.5±9.3) | |
| Present (n = 21) | 127.0 (131.6±10.1) | 0.001 |
*p Value calculated by non‐parametric Mann Whitney U test.
†Compared with stage I.
Discussion
In the present study, we demonstrated that plasma OPN level is a potential new biomarker for gastric cancer development, invasion and survival. To the best of our knowledge, this is the first study to investigate the diagnostic and prognostic values of plasma OPN levels in gastric cancer. The usefulness of elevated plasma OPN level as a diagnostic marker has been reported in a variety of malignancies, including ovarian, colon, breast, lung, prostate, pancreatic and oesophageal cancers.15,16,17,18 Fedarko et al. used competitive ELISA to detect 20 serum samples from different types of cancer, including colon, breast, lung and prostate cancer.17 The sensitivities and specificities of elevated serum OPN level were, respectively: 65.0% and 55.7% for colon cancer; 74.1% and 55.7% for breast cancer; 94.7% and 55.7% for prostate cancer; and 100% and 55.7% for lung cancer.17 In pancreatic cancer, the sensitivity and specificity of elevated serum OPN level were 80% and 97%, respectively.15 In oesophageal cancer, high plasma OPN level was found in 68.9% of patients.18 In ovarian cancer, elevated plasma OPN level showed specificity of 80.4% and sensitivity of 85.4% for late‐stage cancer.16 In the present study, plasma OPN level at a cutoff value of 67.3 ng/ml predicted the occurrence of gastric cancer with sensitivity and specificity of 58.3% and 69.9%, respectively. Combination with H pylori serology did not improve the accuracy of this assay. However, the sensitivity and specificity were 82.1% and 78.0% respectively in the VGH validation cohort. The different stage distribution may contribute to the discrepancy between these two study populations. In the National Taiwan University Hospital cohort, about half the patients were stage III (30.3%) and stage IV (20.5%). In contrast, more than three‐quarters of the VGH validation cohort patients were stage III (51.3%) and stage IV (25.6%). Like other tumour markers, plasma OPN level has higher sensitivity to detect cancer development in a population with more advanced stage disease.
The role of OPN in tumourigenesis can be explained by the multiple functions of OPN in cells.21 Several mechanisms have been proposed through studies using cultured cells. First, it is recognised that OPN has adhesive activity because its receptors all mediate cell adhesion. Second, the ability of cells to migrate may be directly tied to their tumourigenicity and OPN promotes the migration of diverse cells, including monocytes, macrophages and tumour cells, along OPN gradients.22 In addition, OPN‐deficient cells are reported to be hypomotile.23 Third, some experiments suggest that OPN inhibits apoptosis and stimulates survival and growth of cells with inducible OPN,24 or with the addition of OPN to cell culture medium,25 through an interaction with its receptor CD44.26 Fourth, several studies have suggested that OPN increases tumour invasiveness by inducing proteinase, particularly uPA and MMPs, through complex signalling pathways, such as AP‐1 activation, PI3‐kiase/Akt‐dependent or NIK‐dependent NF‐kB activation.27,28,29,30,31 In the present study, we used real‐time RT‐PCR to demonstrate that OPN mRNA expression is significantly higher in gastric cancer tissues compared with surrounding non‐tumour tissues. This observation is compatible with a previous report using a cDNA microarray method in which OPN was overexpressed in gastric cancer tissues.5
We found that elevated plasma OPN not only correlates with gastric cancer occurrence, but also with invasive phenotypes and patient survival. In oesophageal cancer, high plasma OPN level has been reported to correlate with lymph node metastasis and overall survival, but not with depth of invasion.18 In metastatic breast cancer, elevated plasma OPN value has been associated with increased number of metastatic sites and poor survival.32 Similar associations between elevated OPN level and poor survival have been reported in many other kinds of tumours, including prostate cancer,33 adult soft tissue tumours,34 head and neck cancer receiving radiotherapy35 and stage I non‐small‐cell lung cancer.36,37 Consistent with these observations, we found that median plasma OPN concentration is significantly higher in patients with advanced stages, serosal invasion, lymph node metastasis, lymphatic invasion, venous invasion, and liver metastasis. Logistic regression analyses confirmed that elevated plasma OPN level is associated with advanced stages, serosal invasion, lymph node metastasis, lymphatic invasion, venous invasion and liver metastasis. In the VGH validation cohort, we found similar associations. Thus, elevated plasma OPN level seems to represent a metastatic marker for gastric cancer.38,39
In contrast to the numerous reports on the usefulness of plasma OPN as a diagnostic biomarker, the role of plasma OPN as an independent prognostic indicator has not been well investigated. In prostate cancer, plasma OPN level is associated with the presence of metastases, and correlates negatively and independently with patient survival.40 In breast cancer, increased plasma OPN level is the most prognostic value for poor survival.41 However, plasma OPN level is not an independent indicator of survival in oesophageal squamous cell carcinoma.18 In the present study, we found that patients with elevated plasma OPN level have significantly poorer survival rates, especially in the subgroups of patients with invasive phenotypes, such as those with advanced stage, lymph node metastasis or serosal invasion. On Cox multivariate analysis, high OPN level was an independent prognostic factor. However, further studies are needed to validate this observation.
Compared with previous studies and the mean OPN concentration reported by ELISA,15,16,17,18 our study cohorts had lower plasma OPN levels. There are several reasons that may contribute to this discrepancy. First, most previous studies were conducted in Caucasians. Ethnic variation may lead to the different results. Second, many previous results, including ELISA, used mean instead of median to report the distribution of plasma OPN level without examining whether the distribution is Gaussian or not. In a non‐Gaussian distribution, the value of mean tends to be overestimated, especially by extremely high‐value outliers. To resolve this discrepancy, we suggest reporting median instead of mean of plasma OPN concentrations in the future studies. In addition, more studies based on different ethics will be also helpful.
Although plasma OPN level seems to possess potential as a diagnostic and prognostic biomarker in gastric cancer, its potential as a specific marker is doubtful. Several conditions may influence plasma OPN levels, such as age, hyperlipidemia, cardiovascular disease, diabetes, kidney disease and sepsis.21,42 Even in patients known to have cancer, plasma OPN level may be elevated because of non‐cancer causes. In the present study, we found that elderly patients had significantly higher plasma OPN level, which is consistent with a previous report showing significant positive correlation between plasma OPN level and age.42 Further studies using multivariate analyses for these confounders are required to determine whether OPN is an independent prognostic factor.
In addition to the systemic potential confounders, various sources of OPN may influence prognostic value.21 In breast cancer, it has been observed that OPN staining in cancer cells is associated with poor survival; whereas OPN immunopositivity in infiltrating host cells is not associated with patient survival.43 Because OPN is not expressed specifically by tumour cells, but rather by several other tissues, it is suggested that any studies assessing the utility of OPN as a marker of tumour progression consider tissue expression specificity.21 In the present study, we found most OPN immunopositivity in gastric cancer cells, which may partly explain the significant association between plasma OPN expression and patient outcomes in gastric cancer.
There are several limitations to our study. First, selection bias could not be completely excluded in this hospital‐based case–control study, although we tried to enrol cases from different hospitals in three different regions of Taiwan. Future studies based on community populations will be helpful in confirming the diagnostic and prognostic performances of plasma OPN level. Second, we did not study postoperative alteration of plasma OPN levels. To be a good tumour marker, sensitive postoperative change to represent the remission or recurrence of the disease is mandatory. Third, we did not investigate environmental factors, which also play important roles in the occurrence and progression of gastric cancer.
In conclusion, this investigation has validated OPN mRNA overexpression in gastric cancer tissues and localised OPN protein expression mainly in tumour cells. Moreover, elevated plasma OPN level was found to be significantly associated with gastric cancer development, invasive phenotypes and survival. Therefore, we suggest that OPN plays an important role in the tumour development and progression of gastric cancer and that plasma OPN level has the potential to be a useful diagnostic and prognostic factor for gastric cancer.
Acknowledgements
This work was supported by a National Science Council Grant (NSC‐96‐2314‐B‐075A‐015).
Abbreviations
OPN - osteopontin
Footnotes
Competing interest: None.
References
- 1.Gordon D. Luk. Tumors of the stomach. In: Feldman M, ed, Gastrointestinal and liver disease: pathophysiology, diagnosis, management Philadelphia: WB Saunders 2005733–757.
- 2.Roukos D H. Current status and future perspectives in gastric cancer management. Cancer Treat Rev 200026243–255. [DOI] [PubMed] [Google Scholar]
- 3.Kim J P. Surgical results in gastric cancer. Semin Surg Oncol 199917132–138. [DOI] [PubMed] [Google Scholar]
- 4.Miller F H, Kochman M L, Talamonti M S.et al Gastric cancer. Radiologic staging. Radiol Clin North Am 199735331–349. [PubMed] [Google Scholar]
- 5.Lee S, Baek M, Yang H.et al Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays. Cancer Lett 2002184197–206. [DOI] [PubMed] [Google Scholar]
- 6.Fukui R, Nishimori H, Hata F.et al Metastases‐related genes in the classification of liver and peritoneal metastasis in human gastric cancer. J Surg Res 200512994–100. [DOI] [PubMed] [Google Scholar]
- 7.Ue T, Yokozaki H, Kitadai Y.et al Co‐expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer 199879127–132. [DOI] [PubMed] [Google Scholar]
- 8.Sun X J, Zuo W S, Ma H.et al [Expression of osteopontin mRNA and its clinical significance in gastric cancer]. Zhonghua Zhong Liu Za Zhi 200527292–295. [PubMed] [Google Scholar]
- 9.Zhang D T, Yuan J, Yang L.et al [Osteopontin expression and its relation to invasion and metastases in gastric cancer]. Zhonghua Zhong Liu Za Zhi 200527167–169. [PubMed] [Google Scholar]
- 10.Denhardt D T, Guo X. Osteopontin: a protein with diverse functions. FASEB J 199371475–1482. [PubMed] [Google Scholar]
- 11.Ashkar S, Weber G F, Panoutsakopoulou V.et al Eta‐1 (osteopontin): an early component of type‐1 (cell‐mediated) immunity. Science 2000287860–864. [DOI] [PubMed] [Google Scholar]
- 12.Weber G F, Ashkar S, Glimcher M J.et al Receptor‐ligand interaction between CD44 and osteopontin (Eta‐1). Science 1996271509–512. [DOI] [PubMed] [Google Scholar]
- 13.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science 1987238491–497. [DOI] [PubMed] [Google Scholar]
- 14.Rangaswami H, Bulbule A, Kundu G C. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 20061679–87. [DOI] [PubMed] [Google Scholar]
- 15.Koopmann J, Fedarko N S, Jain A.et al Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev 200413487–491. [PubMed] [Google Scholar]
- 16.Kim J H, Skates S J, Uede T.et al Osteopontin as a potential diagnostic biomarker for ovarian cancer. J Am Med Assoc 20022871671–1679. [DOI] [PubMed] [Google Scholar]
- 17.Fedarko N S, Jain A, Karadag A.et al Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res 200174060–4066. [PubMed] [Google Scholar]
- 18.Shimada Y, Watanabe G, Kawamura J.et al Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology 200568285–292. [DOI] [PubMed] [Google Scholar]
- 19.Chen L T, Lin J T, Shyu R Y.et al Prospective study of Helicobacter pylori eradication therapy in stage I(E) high‐grade mucosa‐associated lymphoid tissue lymphoma of the stomach. J Clin Oncol 2001194245–4251. [DOI] [PubMed] [Google Scholar]
- 20.Wu M S, Wu C Y, Chen C J.et al Interleukin‐10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer 2003104617–623. [DOI] [PubMed] [Google Scholar]
- 21.Rittling S R, Chambers A F. Role of osteopontin in tumour progression. Br J Cancer 2004901877–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denhardt D T, Giachelli C M, Rittling S R. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol 200141723–749. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B, Suzuki K, Goldberg H A.et al Osteopontin modulates CD44‐dependent chemotaxis of peritoneal macrophages through G‐protein‐coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol 2004198155–167. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Denhardt D T, Rittling S R. Osteopontin is required for full expression of the transformed phenotype by the ras oncogene. Br J Cancer 200083156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang P L, Cao M, Hicks P. Osteopontin induction is required for tumor promoter‐induced transformation of preneoplastic mouse cells. Carcinogenesis 200 241749–1758. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y H, Huang C J, Chao J R.et al Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin‐3 or granulocyte‐macrophage colony‐stimulating factor. Mol Cell Biol 2000202734–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaulian E, Karin M. AP‐1 as a regulator of cell life and death. Nat Cell Biol 20024E131–E136. [DOI] [PubMed] [Google Scholar]
- 28.Tuck A B, Arsenault D M, O'Malley F P.et al Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene 1999184237–4246. [DOI] [PubMed] [Google Scholar]
- 29.Das R, Mahabeleshwar G H, Kundu G C. Osteopontin induces AP‐1‐mediated secretion of urokinase‐type plasminogen activator through c‐Src‐dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem 200427911051–11064. [DOI] [PubMed] [Google Scholar]
- 30.Rangaswami H, Bulbule A, Kundu G C. Nuclear factor‐inducing kinase plays a crucial role in osteopontin‐induced MAPK/IkappaBalpha kinase‐dependent nuclear factor kappaB‐mediated promatrix metalloproteinase‐9 activation. J Biol Chem 200427938921–38935. [DOI] [PubMed] [Google Scholar]
- 31.Rangaswami H, Bulbule A, Kundu G C. JNK1 differentially regulates osteopontin‐induced nuclear factor‐inducing kinase/MEKK1‐dependent activating protein‐1‐mediated promatrix metalloproteinase‐9 activation. J Biol Chem 200528019381–19392. [DOI] [PubMed] [Google Scholar]
- 32.Singhal H, Bautista D S, Tonkin K S.et al Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res 19973605–611. [PubMed] [Google Scholar]
- 33.Forootan S S, Foster C S, Aachi V R.et al Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer 20061182255–2261. [DOI] [PubMed] [Google Scholar]
- 34.Bramwell V H, Tuck A B, Wilson S M.et al Expression of osteopontin and HGF/Met in adult soft tissue tumors. Cancer Biol Ther 200541336–1341. [DOI] [PubMed] [Google Scholar]
- 35.Overgaard J, Eriksen J G, Nordsmark M.et al Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double‐blind placebo‐controlled trial. Lancet Oncol 20056757–764. [DOI] [PubMed] [Google Scholar]
- 36.Donati V, Boldrini L, Dell'Omodarme M.et al Osteopontin expression and prognostic significance in non‐small cell lung cancer. Clin Cancer Res 2005116459–6465. [DOI] [PubMed] [Google Scholar]
- 37.Boldrini L, Donati V, Dell'Omodarme M.et al Prognostic significance of osteopontin expression in early‐stage non‐small‐cell lung cancer. Br J Cancer 200593453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber G F. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta 2001155261–85. [DOI] [PubMed] [Google Scholar]
- 39.Chambers A F, Tuck A B. Ras‐responsive genes and tumor metastasis. Crit Rev Oncog 1993495–114. [PubMed] [Google Scholar]
- 40.Hotte S J, Winquist E W, Stitt L.et al Plasma osteopontin: associations with survival and metastasis to bone in men with hormone‐refractory prostate carcinoma. Cancer 200295506–512. [DOI] [PubMed] [Google Scholar]
- 41.Bramwell V H, Doig G S, Tuck A B.et al Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res 2006123337–3343. [DOI] [PubMed] [Google Scholar]
- 42.Takemoto M, Tada K, Nakatsuka K.et al [Effects of aging and hyperlipidemia on plasma osteopontin level]. Nippon Ronen Igakkai Zasshi 199936799–802. [DOI] [PubMed] [Google Scholar]
- 43.Tuck A B, O'Malley F P, Singhal H.et al Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer 199879502–508. [DOI] [PubMed] [Google Scholar]