Abstract
Background
The potential of endoscopic mucosal resection (EMR) for treating flat dysplastic lesions in chronic ulcerative colitis (CUC) has not been addressed so far. Historically, such lesions were referred for colectomy. Furthermore, there are only limited data to support endoscopic resection of exophytic adenoma‐like mass (ALM) lesions in colitis.
Aims
To evaluate the safety and clinical outcomes of patients with colitis undergoing EMR for Paris class 0–II and class I ALM compared with sporadic controls. Secondary aims were to re‐evaluate the prevalence, anatomical “mapping” and histopathological characteristics of both Paris class 0–II and class I lesions in the context of CUC.
Methods
Prospective clinical, pathological and outcome data of patients with colitis‐associated Paris class 0–II and Paris class I ALM treated with EMR (primary end points being colorectal cancer development, resection efficacy, metachronous lesion rates and post‐resection recurrence rates) were compared with those of sporadic controls.
Results
204 lesions were diagnosed in 169 patients during the study period: 167 (82%) diagnosed at “entry” colonoscopy, and 36 (18%) diagnosed at follow‐up. 170 ALMs, 18 dysplasia‐associated lesion masses (DALMs) and 16 cancers were diagnosed. A total of 4316 colonoscopies were performed throughout the study period (median per patient: 6; range: 1–8). The median follow‐up period for the complete cohort was 4.1 years (range: 3.6–5.21). 1675 controls were included from our prospective database of patients without CUC who had undergone EMR for sporadic Paris class 0–II and snare polypectomy of Paris type I lesions from 1998 onwards, and were considered to be at moderate to high lifetime risk of colorectal cancer. 3792 colonoscopies were performed throughout the study period in this group (median per patient: 4; range: 1–7). The median follow‐up period was 4.8 years (range: 2.9–5.2). No statistically significant differences were observed between the CUC study group and controls with respect to age, sex, median number of colonoscopies per patient, median follow‐up duration, post‐resection complications, median lesional diameter or interval cancer rates. However, there was a significant between‐group difference regarding the prevalence of Paris class 0–II lesions in the CUC group (82/155 (61%)) compared with controls (285/801 (35%); χ2 = 31.13; p<0.001). Furthermore, recurrence rates of lateral spreading tumours were higher in the colitis cohort (1/7 (14%)) than among controls (0/10 (0%); p = 0.048 (95% CI 11.64% to 40.21%)).
Conclusions
Flat DALM, similarly to Paris class I ALM, can be managed safely by EMR in CUC. A change in management paradigm to include EMR for the resection of flat dysplastic lesions in selected cases is proposed.
Previous studies have demonstrated that dysplasia in chronic ulcerative colitis (CUC) can be morphologically heterogeneous, and occurs in endoscopically “normal” flat mucosa (plaque‐like), or as an elevated mass.1 The terms DALM (dysplasia‐associated lesion mass) and ALM (adenoma‐like mass) attempt to differentiate those lesions, which resemble sporadic adenomas and may therefore be amenable to endoluminal resection. Data from Rubin et al2 and Engelsgjerd et al3 now suggest that polypoid lesions defined as ALMs (absence of adjacent flat mucosal dysplasia) can be managed conservatively with polypectomy, as the subsequent risk of dysplasia and cancer is only 4% after a median of 82 months of endoscopic follow‐up. However, no studies to date have addressed the safety and efficacy of endoscopic mucosal resection (EMR) for the endoluminal treatment of Paris type 0–II lesions in CUC; three prospective studies to date have demonstrated a prevalence similar to that of non‐CUC surveillance cohorts.4,5,6 Delineating the validity of EMR in the context of CUC could, therefore, fundamentally change the management paradigm of Paris type 0–II dysplasia to that of endoscopy‐based strategies rather than surgical resection.
Primary and secondary study end points
The primary aim of this study was to evaluate the safety and outcome of EMR for Paris class 0–II and class I ALM in patients with CUC, compared with a control group of patients without CUC who underwent resection of sporadic Paris class 0–II/I lesions endoscopically and were considered to be at moderate to high lifetime risk of colorectal neoplasia. The clinical end points were post‐resection recurrence rates, resection‐related complications and interval cancer formation at medium‐term follow‐up. The secondary aims were to re‐evaluate the prevalence, anatomical mapping and histopathological characteristics of both Paris class 0–II and class I lesions in the context of CUC.
Patients and methods
CUC study group
From June 2000 to April 2006, 736 patients with clinically quiescent, longstanding CUC (>8 years) were recruited from the Inflammatory Bowel Disease Clinic at the Royal Hallamshire Hospital, Sheffield, UK. All patients were colonoscoped using the Olympus C240Z magnification colonoscope (Olympus Keymed, Southend‐on‐sea, UK) by a single endoscopist (DPH). Written informed consent was obtained from all participants, with ethical approval granted from the South Sheffield ethics committee (ref: SS/01/165). Study inclusion criteria were histologically verified pan‐CUC, with a concurrent colitis activity index <8.
Exclusion criteria for the study are summarised in box 1.
Box 1 Exclusion criteria for the study
Non‐correctable coagulopathy (prothrombin time >14 s/platlet count <90×109/l)
Pregnanacy
Known intraepithelial neoplasia or colorectal cancer at index endoscopy
Insufficient bowel preparation
Previous documented allergy to indigo carmine or crystal violet solution
“Lead pipe” colorectal fibrosis
Multiple inflammatory/pseudo polyps
Endoscopic technique
Fluid residue was aspirated at index intubation to maximise mucosal views. Following caecal intubation, an Olympus PW‐5L‐1 (Keymed, Southend‐on‐sea, UK) colonic diffusion catheter was used to apply 25 ml of 0.5% indigo carmine (IC) solution to each 10 cm segment before biopsy. Conventional quadrantic biopsies were taken every 10 cm throughout the colorectum in addition to targeted biopsies of abnormal mucosa. The anatomical site and morphological class of identified lesions were then recorded.
Endoscopic classification of lesions
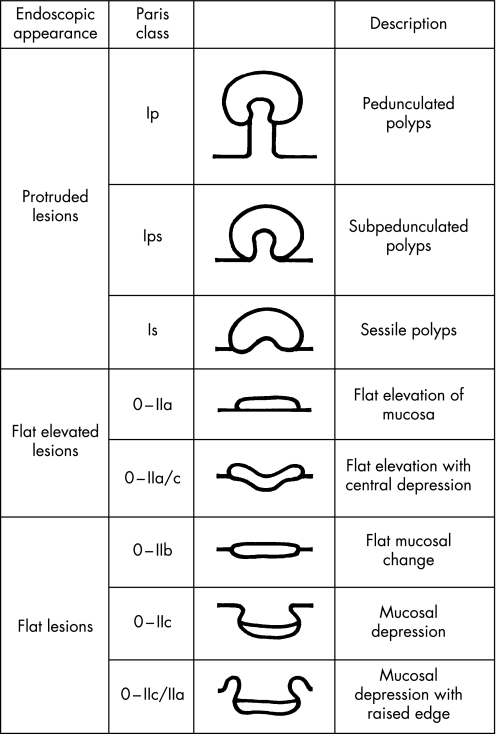
Lesion morphology was documented using the Paris classification consensus workshop guidelines.7 Using Paris criteria, superficial lesions were classified according to sub‐types of type 0 morphology—namely, 0–I, polypoid; 0–II, non‐polypoid and non‐excavated; 0–III, non‐polypoid with frank ulceration. Type 0–II lesions included three distinct subgroups (0–IIa, elevated; 0–IIb, completely flat with the mucosa; 0–IIc, slight depression without an ulcer crater). A centrally depressed lesion was classified as type 0–IIc+Iia, in contrast with a primary elevated lesion with a central depression at its apex (0–IIa+IIc)—in the latter class, the relative depression, as a rule, does not extend below the level of the adjacent normal mucosa. A simplified schematic summary of the Paris classification is shown in fig 1.
Figure 1 Schematic summary of the Paris morphological classification system of colorectal lesions.
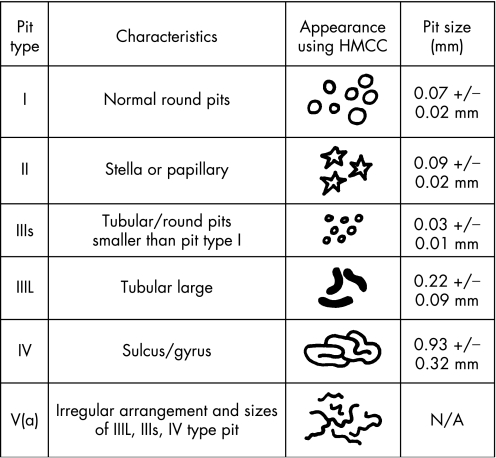
Following morphological classification using IC chromoscopy, all identified lesions underwent high‐magnification examination using high‐magnification chromoscopic colonoscopy, (HMCC; ×100 normal resolution). The surface pit pattern was classified according to the modified Kudo criteria, as summarised in fig 2.8 Following HMCC imaging of any localised lesion, pan‐HMCC of the adjacent mucosa was also performed to establish the presence or absence of adjacent flat dysplastic change, as evidenced by crypt type IIIs/IIIL/IV/V.
Figure 2 The modified Kudo criteria for the classification of colorectal crypt architecture in vivo using high‐magnification chromoscopic colonoscopy.
Endoscopic resection method
All identified lesions were biopsied, removed by snare polypectomy, EMR, endoscopic submucosal dissection, or referred for surgical resection. The mucosa adjacent to the identified lesion was also biopsied (using HMCC targeting). Adjacent mucosal tattoos were placed in all cases. The following clinical criteria9 were used to guide the appropriate diagnostic or therapeutic intervention:
Endoscopic exclusion criteria for EMR/ESD—surgical referral
Lesions showing a Kudo type V(n) pit pattern due to the possibility of deep submucosal invasion10,11
Lesions showing asymmetric “lift” at submucosal injection
Lesions where the adjacent mucosa was found to have flat neoplastic change on HMCC—that is, the endoscopic criterion for DALM.
Lesions excluded from EMR were cold biopsied alone and marked with a submucosal tattoo (“spot” endoscopic marker (Ref: GIS‐44, Camp Hill, PA 17011)) applied adjacent to the lesion, to facilitate future localisation, if required, and surgical referral made.
Post‐resection evaluation
Following tissue retrieval (Roth net, US Endoscopy, Mentor, Ohio, USA), the colorectal mucosal defect was again washed with saline and then reassessed using the high‐magnification mode, following a second dye spray of indigo carmine at the cut margin. Horizontal margins showing a type I pit pattern were used as evidence of complete endoscopic resection. Clear vertical margins were predicted endoscopically if no remnant tissue with a discernible pit pattern was present in the resection crater. If a remnant tissue was identified, then the EMR was extended (piecemeal approach) and argon plasma coagulation ablation applied to the horizontal cut margins. Where piecemeal EMR failed to establish a complete resection, surgical referral was made. Complications were defined as early (within 24 h after the procedure) or late (>24 h after the procedure).
Histopathological analysis
A single designated specialist gastrointestinal pathologist (SSC) examined all specimens, with a second independent opinion sought (as per the British Society of Gastroenterology (BSG) guidelines) for dysplastic interpretation.12 The tissue was immediately fixed in 10% buffered formalin solution, embedded in paraffin wax and subsequently stained with H&E. Dysplasia was defined, according to the modified Vienna criteria, as either low grade (LGD) or high grade (HGD).13 Invasive neoplasia (IN) was defined as neoplastic cellular proliferation extending into the deep submucosal layer 3, or beyond, to the muscularis propria.13 Early colorectal cancer was defined as the presence of intra‐ or superficial submucosal carcinoma, with no vertical extension into the submucosal layer 3 or the muscularis propria.13
Endoscopic resection specimens and adjacent mucosal biopsy specimens were sent for analysis in two separate formalin pots. This enabled the histological differentiation of DALM and ALM. For patients undergoing colectomy, the presence of adenocarcinoma and/or concurrent Paris 0–II/I dysplastic lesions with anatomical site was documented.
Post‐resection surveillance protocol
Repeat chromoscopic colonoscopy was performed at 1, 3 and 6 months after index resection, with biannual colonoscopies thereafter. Recurrent neoplastic disease was defined, as per Higaki criteria,14 as the presence of tumour at the previous resection site, or evidence of tumour with associated fold convergence or tumour (in the absence of fold convergence) 1–2 mm adjacent to the EMR mucosal scar. If recurrent neoplastic disease was identified at follow‐up colonoscopy, a further extended EMR was performed using the same resection technique as described above. Patients with histology suggestive of invasive disease (submucosal layer 2, with infiltration), adjacent mucosal dysplastic change at either index or follow‐up EMR, or dysplasia within sequential quadrantic or targeted biopsies were referred for surgical resection and excluded from long‐term endoscopic follow‐up.
Control group
Controls were taken from our prospective database of patients without CUC who had undergone EMR of Paris class 0–II and snare polypectomy of Paris type I lesions since 1998, and were considered to be at moderate to high lifetime risk of colorectal neoplasia. This cohort had been the subject of previous published data by our group, and had undergone an identical augmented post‐EMR surveillance protocol as described for the CUC study group.15,16,17
Statistical analysis
For the purpose of this study, we set the α and β levels to 0.05 and 0.2, reflecting an overall power of 0.9. Using HMCC‐assisted EMR for the resection of sporadic Paris 0–II class lesions (published in our previous series15,16,17,18), we defined an overall complication rate of 0.5%, and the medium‐term recurrence rate of 3% indicated that our sample size was sufficient to detect a 5–6% difference in post‐resection recurrence and complication rates in the CUC study group compared with controls. Between‐group differences were analysed using χ2 tests, with appropriate use of the continuity correction where indicated.
Results
CUC study group
A total of 736 patients fulfilled the entry criteria for the study, with 24 being excluded from final analysis (13 lost to follow‐up, 8 secondary to incomplete colonoscopy during the surveillance programme and 3 due to failed bowel preparation on three consecutive occasions). Outcome data from 712 patients were thus obtained (413 (58%) men/299 (42%) women; mean age at entry 58.5 years (range: 21–74)). A total of 4316 colonoscopies were performed throughout the study period (median per patient: 6; range: 1–8). The median follow‐up period for the complete cohort was 4.1 years (range: 3.6–5.21), with the median duration of CUC being 19.5 years (range: 8–61). In all, 5 (0.7%) patients had a concurrent diagnosis of primary sclerosing cholangitis complicating CUC. During the study period, 204 lesions were diagnosed in 169 patients. Of these, 167 (82%) lesions were diagnosed at index “entry” colonoscopy, and 36 (18%) lesions at follow‐up (phases 2–11). A total of 170 ALMs, 18 DALMs and 16 cancers were diagnosed.
Index colonoscopy findings
Table 1 summarises the findings at index colonoscopy of the CVC study group.
Table 1 Summary of endosopically detected lesions at index colonoscopy of the CUC study group.
| Lesion no | Paris class | Anatomical location | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right colon | Left colon | |||||||||
| 0–II | I | LST | 0–II | I | LST | 0–II | I | LST | ||
| ALM | 135 | 82 | 46 | 7 | 52 | 10 | 3 | 30 | 36 | 4 |
| (61%) | (34%) | (5%) | (63%) | (22%) | (43%) | (37%) | (78%) | (57%) | ||
| DALM | 18 | (14) | (4) | (0) | (14) | (1) | (0) | (0) | (3) | (0) |
| (77%) | (23%) | (0%) | (100%) | (25%) | (0%) | (0%) | (75%) | (0%) | ||
| Cancer | 14 | (9) | (5) | (0) | (8) | (0) | (0) | (1) | (5) | (0) |
| (64%) | (36%) | (0%) | (88%) | (0%) | (0%) | (12%) | (100%) | (0%) | ||
| Total | 167 | (105) | (55) | (7) | ||||||
ALM, adenoma‐like mass; DALM, dysplasia‐associated lesion mass; LST, lateral spreading tumour.
Values are shown as n (%).
Adenoma‐like mass
A total of 112 patients had endoscopically resectable ALMs, equating to a prevalence of 15.7%. A total of 135 ALMs were detected in 112 patients, of which 82 (61%) were of Paris type 0–II morphology, 46 (34%) of Paris type I and 7 (5%) were classified as LST (4 G type and 3 F (NG)). Of the Paris 0–II ALMs, 52 (63%) were located proximal to the splenic flexure, compared with 10 (22%) of Paris type I lesions. Of the 4 G‐type LSTs, 3 (75%) were located in the rectum, with 1 in the proximal sigmoid, compared with all the 3 caecal LST NG lesions being located in the caecum. The median diameters of the Paris type 0–II, Paris I and LST lesions were 8 mm (range: 2–24), 15 mm (range: 4–38) and 35 mm (range: 18–48), respectively.
Dysplasia‐associated lesional mass
Eighteen DALMs (defined as lesions with adjacent flat mucosal dysplasia) were detected in 18 patients (2.5%) of the cohort. HMCC in vivo imaging correctly differentiated these lesions from ALM in all 18 cases. Of these lesions, 14 (77%) were of Paris 0–II morphology and 4 (23%) were of Paris I morphology (median diameter 17 mm (range: 1–26) and 16 mm (range: 6–24), respectively). All 14 (100%) and 1 (25%) of the Paris type 0–II and I DALMs were located proximal to the splenic flexure.
Endoscopically detectable carcinoma
A total of 14 adenocarcinomas were detected endoscopically in 13 patients (1.8%), of which 9 (64%) and 5 14 (36%) were classified as Paris 0–II and I, respectively. Anatomically, 8 of 9 (88%) and 0 of 5 (0%) of the Paris class 0–II and class I cancers, respectively, were located proximal to the splenic flexure. Of the 9 Paris class 0–II adenocarcinomas, 4 (44%) showed a type 0–IIa/c morphology and 2 (22%) showed a primary 0–IIc configuration.
Dysplasia in non‐circumscribed lesional mucosa
A total of 59 (5%) patients had dysplasia confirmed by biopsy in specimens taken according to the BSG guidelines (10 cm serial quadrantic). Of these, 30 (51%) patients had unifocal LGD, whereas 14 (23%), 12 (20%) and 3 (5%) patients had multi‐focal LGD, unifocal HGD and multi‐focal HGD, respectively. The clinical outcome data of this cohort are discussed below.
Clinical outcomes
Endoscopic resection group
In all, 135 lesions from 112 patients fulfilled the inclusion criteria for endoscopic resection at index colonoscopy.
Flat (Paris 0–II) dysplastic lesions
A total of 82 flat Paris 0–II lesions underwent EMR; 76 (93%) were performed using an en bloc resection, and 3 (3.5%) required a piecemeal dissection technique. Another 3 (3.5%) lesions could not be resected endoscopically, because of non‐lifting at submucosal injection in 2 cases, and, in the third case, because of retrograde fold extension at the hepatic flexure, making EMR technically impossible. These three cases were referred for pan‐proctocolectomy. Postoperative histopathology showed adenoma (LGD) in both cases that failed to lift, and tubulovillous adenoma with focal HGD in the remaining case. No synchronous colorectal neoplastic lesions were reported in these colectomy specimens. There were no perforations in this group, but one case of delayed bleeding required transfusion and endoscopic mucosal defect clip closure. However, 2 (2.4%) patients developed Higaki criteria for recurrence at 3 months after resection, requiring an extended EMR. Recurrence rate in this group was 2.4%, at a median follow‐up of 4.05 years (range: 3.6–5.1). No further flat dysplasia or carcinoma was diagnosed in this cohort throughout the study period.
Flat (LST) lateral spreading dysplastic lesions
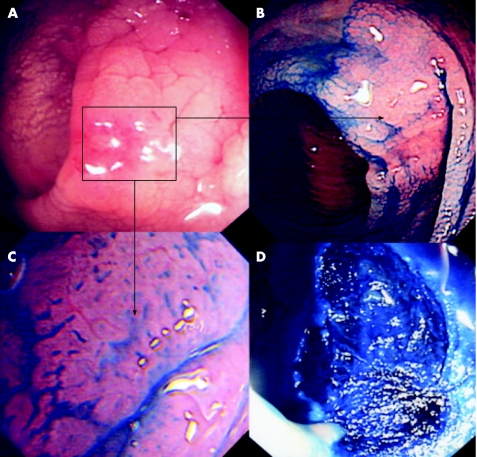
Seven LSTs were resected in seven patients at index colonoscopy. Endoscopic submucosal dissection (ESD) using the cap‐assisted method was performed for all four LST‐G type lesions (three rectum/one recto‐sigmoid), with the extended piecemeal EMR technique being used for the three caecal LST‐NG lesions. There were no perforations or haemostatic complications in this group. One caecal LST fulfilled the Higaki criteria for recurrence at 3 months after index resection, requiring a second EMR. Recurrence rate in this group was 14%, at a median endoscopic follow‐up of 4.1 years (range: 3.8–4.6). No further flat dysplasia or carcinoma was diagnosed in this cohort at follow‐up. A clinical example is shown in figs 3 and 4.
Figure 3 (A) Conventional endoscopic appearance of the distal sigmoid colon in a 52‐year‐old woman (duration of colitis, 15 years). There is marked mucosal oedema, vascular net loss, nodularity and focal central erythema. (B) Mucosal appearance after chromoscopy with indigo carmine 0.5%. A lateral spreading tumour (LST‐NG type) is now clearly delineated. (C) High‐magnification chromoscopic images of the lesion at magnification ×100. A type II/IIIL crypt pattern is evident (neoplastic/non‐invasive). (D) Mucosal defect after post endoscopic dissection, with successful vertical plane dissection to the muscularis layer—see the associated blue stranding of the muscularis layer at indigo carmine chromoscopy.
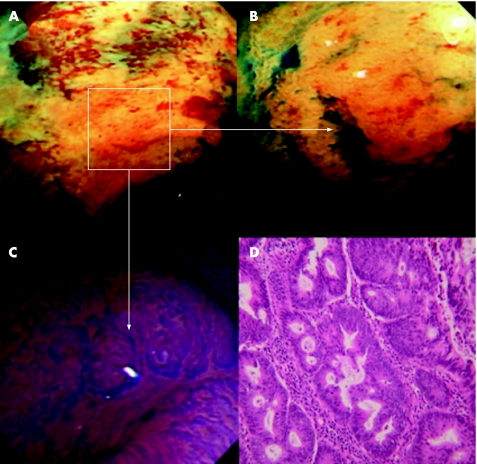
Figure 4 (A) Conventional endoscopic appearances of the distal ascending colon in a 66‐year‐old man (duration of colitis >10 years). There is marked mucosal pallor (highlighted area) with a nodular centre. (B) The highlighted segment following indigo carmine 0.5% chromoscopy. A clearly delineated Paris 0–IIa/c lesion is now seen. (C) High‐magnification imaging of the area that is centrally depressed using crystal violet solution 0.5% (magnification ×100). The crypt pattern is a Kudo V(n) neoplastic/invasive architecture. (D) Postoperative high‐power histopathology section. There is an invasive adenocarcinoma arising within an area of high‐grade dysplasia.
Exophytic (Paris I) dysplastic lesions
A total of 46 Paris type I lesions underwent simple polypectomy, all using a single pass en bloc resection. There were no reported complications, with a single recto‐sigmoid recurrence at 6 months after resection requiring further polypectomy. Recurrence rate was 2%, at a median follow‐up of 4 years (range: 3.7–4.65). No patients in this group developed further discrete flat mucosal dysplasia while undergoing endoscopic follow‐up.
Post‐endoscopic resection histopathology
Focal HGD was detected in 15 of 82 (18%) and 4 of 46 (8%) of the Paris class 0–II and class I lesions, respectively. Low‐grade dysplasia was reported in the remaining 67 of 82 (82%) Paris class 0–II lesions and in 42 of 46 (92%) of the Paris class I group. There were no reported carcinomas or HGD appearances with accompanying vertical infiltration beyond 1000 μm in either group. All LSTs showed LGD villous appearances. Paris class 0–II lesions were more likely to exhibit focal HGD, irrespective of lesional diameter, than Paris class I lesions (18% vs 8%, respectively; p<0.01). Focal HGD was also more commonly observed in Paris class 0–II lesions proximal to the splenic flexure (13/15 (86%)) than in the distal colon (2/15 (14%); p<0.01).
Surgical referral group
DALM
All 18 patients fulfilling the endoscopic criteria for DALM at index colonoscopy were referred for pan‐proctocolectomy following clinical consult. Within this cohort, 7 (39%) patients had confirmed adenocarcinoma within the resection specimen, of which 4 (57%) were remote to the anatomical DALM site. There were no synchronous colorectal neoplasias within this group.
Carcinoma
Of the 14 patients with carcinomas that were detected endoscopically at entry, 13 consented to pan‐proctocolectomy (one patient refused further intervention except for palliative stenting, and died 1 month later). In these 13 patients, 13 adenocarcinomas were present in the operative colectomy specimens, of which carcinomas of patients 1, 2, 3 and 7 belonged to stages T1, 2, 3 and 4, respectively.
Dysplasia in non‐circumscribed lesional mucosa
All of the 59 (5%) patients who had IN confirmed by biopsy, in random quadrantic 10 cm specimens taken according to the BSG CUC surveillance guidelines, were counselled in clinic and offered prophylactic pan‐proctocolectomy.12 One patient in the unifocal LGD group (1/30 (3%)) elected for pan‐proctocolectomy, with the remaining 29/30 (97%) patients electing for continued surveillance according to the entry endoscopic surveillance protocol. Postoperative histopathology revealed no evidence of carcinoma, and no metachronous IN lesions developed in the remaining 29 patients undergoing colonoscopic surveillance (median follow‐up 3.8 years (range: 3.5–4.7)). Of the 14 patients with multi‐focal LGD, 12 (86%) elected for pan‐proctocolectomy and 2 (14%) for ongoing colonoscopic surveillance. Postoperative colectomy revealed an 8 mm Paris class 0–III lesion in the ascending colon in one patient, which was not diagnosed at index colonoscopy. Postoperative histopathology confirmed a T2/N0 well‐differentiated adenocarcinoma. All 12 patients with unifocal HGD elected for pan‐proctocolectomy. Of these, 6 (50%) patients had endoscopically detected DALM at index colonoscopy. Another 3 (25%) patients had evidence of adenocarcinoma at pan‐proctocolectomy, and 6 (50%) had endoscopic detectable carcinoma at index colonoscopy. All 3 (100%) patients with multifocal HGD had endoscopically detectable carcinoma at index colonoscopy. These data are summarised in table 2.
Table 2 Summary of outcome data for patients with dysplasia in non‐circumscribed lesional mucosa.
| Dysplasia | Patient number | Surgical findings | Endoscopic surveillance | ||||
|---|---|---|---|---|---|---|---|
| Number | Cancer | Colitis only | Number | Cancer | Metachronous IN to study conclusion | ||
| UF‐LGD | 30 (51%) | 1 | 0 | 1 | 29 | 0 | 0 |
| MF‐LGD | 14 (23%) | 12 | 1 | 13 | 2 | 0 | 0 |
| UF‐HGD | 12 (20%) | 12 | 9 | 0 | 0 | 0 | 0 |
| MF‐HGD | 3 (5%) | 3 | 3 | 0 | 0 | 0 | 0 |
| Total | 59 (100%) | 28 | 13 | 14 | 31 | 1 | 4 |
IN, invasive neoplasia; MF‐HGD, multifocal high‐grade dysplasia; MF‐LGD, multifocal low‐grade dysplasia; UF‐HGD, unifocal high‐grade dysplasia; UF‐LGD, unifocal low‐grade dysplasia.
Follow‐up colonoscopy findings
Of the 655 (91%) patients entering phase 1 surveillance, 36 metachronous lesions were diagnosed in 23 patients. Morphologically, these lesions comprised 27 Paris class 0–II lesions and 9 Paris class I. There were no metachronous LSTs diagnosed at follow‐up. Median follow‐up in this group was 3.1 years (range: 2.8–3.4). These data are summarised in table 3.
Table 3 Summary of endosopically detected lesions at follow‐up colonoscopy in the chronic ulcerative colitis study group.
| Lesion number | Paris class | Anatomical location | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right colon | Left colon | |||||||||
| 0–II | I | LST | 0–II | I | LST | 0–II | I | LST | ||
| ALM | 35 | 26 (74%) | 9 (25%) | 0 (0%) | 18 (69%) | 0 (0%) | 0 (0%) | 8 (31%) | 9 (100%) | 0 (0%) |
| DALM | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cancer | 1 | 1 (1%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 8 (12%) | 9 (100%) | 0 (0%) |
| Total | 36 | 27 | 9 | 0 | ||||||
ALM, adenoma‐like mass; DALM, dysplasia‐associated lesion mass; LST, lateral spreading tumour.
Flat (Paris 0–II) dysplastic lesions
In all, 27 Paris class 0–II lesions were diagnosed in 12 patients (median diameter 8.5 mm (range: 2–12)). Of these, 25 (93%), 9 (33%), 1 (3%) and 1 (3%) lesions were detected at follow‐up phases 2, 3, 5 and 6, respectively. In all, 19 (70%) of the lesions were located in the right and 8 (30%) in the left‐hemi‐colon. The EMR criteria for ALM were fulfilled in 26 (96%) of the lesions, with one caecal 8 mm Paris class 0–IIa/c lesion with a central type V(n) crypt pattern being referred for pan‐proctocolectomy. An adenocarcinoma with submucosal layer 2 invasion was found in the postoperative histopathological specimen. One patient developed subcutaneous emphysema post‐resection of a Paris class 0–IIa caecal LGD ALM, which was managed conservatively with intravenous antibiotic therapy.
Post‐EMR resection histopathology in this group showed focal HGD adenoma in 1 (4%), serrated adenoma in 1 (4%) and LGD adenoma in 24 (88%) cases. All lesions were resected en bloc with verified R0 resection margins at histopathology. A single Paris class 0–II lesion (index resection status LGD) recurred during phase 6 surveillance, which required an extended EMR. Recurrence rate was, therefore, 3.8% in this cohort. No patients in this group developed further discrete flat mucosal dysplasia or carcinoma at follow‐up.
Exophytic (Paris I) dysplastic lesions
A further nine Paris class I ALMs were diagnosed in four patients (median diameter 6.5 mm (range: 2–14), with 4 (44%) and 5 (56%) diagnosed at surveillance phases 2 and 4, respectively). All 9 (100%) lesions were located within the left‐hemi‐colon, and showed LGD atypia only at post‐resection histopathology. There was no endoscopic detectable recurrence or complications in this group. No flat dysplasia or carcinoma was detected at follow‐up.
Control group
Controls were selected from our prospective database of patients without CUC who had undergone EMR of Paris class 0–II and snare polypectomy of Paris class I lesions from 1998 to June 2006, and considered to be at moderate to high lifetime risk of colorectal neoplasia. These data had previously been published by our group. All patients underwent diagnostic targeted chromoscopy to unmask circumscribed Paris class 0–II lesions, and also underwent an identical endoscopic resection and surveillance protocol as detailed for the CUC group in this study (see Patients and methods).
Summary of control group characteristics/demographic data
A total of 1675 patients acted as controls (1022 (61%) men and 653 (39%) women), median age at entry was 62 years (range 22–84). A total of 3792 colonoscopies were performed throughout the study period in this group (median per patient 4; range: 1–7). The median follow‐up period for the complete cohort was 4.8 years (range: 2.9–5.2). In total, 801 of the lesions eligible for endoscopic resection were diagnosed and resected throughout the duration of the study. Of the 801 lesions, 609 (76%) were diagnosed at index “entry” colonoscopy, and 192 (24%) at follow‐up (phase 2–11). A total of 27 (3.4%) lesions recurred at follow‐up.
Flat (Paris 0–II) dysplastic lesions
A total of 285 Paris class 0–II lesions, including 6 LST‐G and 4 LST‐NG lesions, underwent EMR (median diameter: 9.5 mm; range: 1–46 mm), of which 214 (75%) were LGD adenomas, 66 (23%) were HGD adenomas and 5 (1.7%) were intramucosal carcinomas. Of these Paris class 0–II lesions, 202 (71%) were anatomically proximal to the splenic flexure, with 83 (29%) being present in the left colon. There were no perforations or haemostatic complications in this group.
Exophytic (Paris I) dysplastic lesions
Paris class I lesions accounted for 466 of 801 (58%) lesions in the control group, with 407 (87%) of these being LGD adenomas, and 58 (12%) showing focal HGD. There was one (<1%) case of invasive carcinoma. Of the 466 lesions, 383 (71%) were anatomically located distal to the splenic flexure, and 85 (19%) were located proximal. The median lesional diameter was 16 mm (range: 1–32). There were two cases of delayed bleeding and two perforations in this group.
Comparative data between the CUC group and controls
There were no statistically significant differences observed between the CUC study group and controls with respect to age, sex, median number of colonoscopies per patient, median follow‐up duration, post‐resection complications, median lesional diameter or interval cancer rates. However, there was a significant between‐group difference regarding Paris class 0–II lesion prevalence in the CUC group (82/155 (61%)) compared with controls (285/801 (35%); χ2 = 31.13; p<0.001). Furthermore, LST recurrence rates were higher in the CUC cohort (1/7 (14%)) than among controls (0/10 (0%); p = 0.048 (95% CI −11.64% to 40.21%)). However, small numbers with a wide 95% CI limit the extrapolation of these data to clinical practice until further reports from multicentre randomised controlled trials have been carried out. The complete comparative dataset for the above parameters is summarised in table 4.
Table 4 Comparative data and end point comparisons between the chronic ulcerative colitis group and sporadic controls.
| Age (years) | Sex (female) | Median no. colonoscopies/patient | Median f/u duration—years | Post‐resection recurrence rate | Median lesion diameter (mm) | Post resection complications | Interval cancers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cohort | Paris 0–II | Paris I | LST | Total cohort | Paris 0–II | Paris I | LST | Paris 0–II | Paris I | LST | Bleed | Perforation | Paris 0‐II | Paris I | Total | ||||
| CUC group | 58 (21 to 74) | 413/712 58% | 6 (1 to 8) | 4.1 (2.8 to 5.4) | 4.05 (3.6 to 5.1) | 4.0 (3.7 to 4.65) | 4.1 (3.8 to 4.6) | 5/170 2.94% | 3/108 2.7% | 1/55 1.8% | 1/7 14% | 8 (2 to 24) | 15 (4 to 38) | 35 (18 to 48) | 1/170 0.58% | 1/170 0.58% | 1 0.14% | 0 0% | 1 0.14% |
| Control group | 62 (22 to 84) | 1022/1675 61% | 4 (1 to 7) | 4.8 (2.9 to 5.2) | 4.9 (2.8 to 6.1) | 4.5 (3.8 to 4.9) | 4.0 (2.1 to 5.0) | 27/801 3.4% | 7/275 2.6% | 10/466 2.14% | 0/10 0% | 9.5 (2 to 22) | 16 (1 to 32) | 29 (20 to 46) | 2/801 0.25% | 2/801 0.25% | 2 0.24% | 0 0% | 2 0.24% |
| p Value | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | p = 0.048 | NS | NS | NS | NS | NS | NS | NS | NS |
CUC, chronic ulcerative colitis; LST, lateral spreading tumour; NS, not significant.
Discussion
This is the first study to address the prevalence, histopathological features and endoscopic management of flat dysplastic lesions in CUC using EMR. Our results have the potential to change the current management paradigm of pan‐proctocolectomy to that of endoluminal resection in carefully selected cases. We have also shown that using new endoscopic techniques such as HMCC and mucosal chromoscopy significantly enhances the detection and characterisation of IN lesions in CUC and may represent the new “gold standard” surveillance tool in this group.
The endoscopic and histopathological interpretation of colorectal dysplastic lesions complicating CUC has been a subject of much controversy.19 The principal problem, endoscopically, is the reliable differentiation of sporadic ALM and DALM, and identification of morphologically subtle flat dysplastic lesions (Paris 0–II) that often demonstrate poor prognostic histopathological characteristics.20 Previous molecular and histopathological series have also been unable to reliably distinguish between these discrete entities, which further adds to the clinician's difficulty when selecting patients at high risk of colorectal cancer requiring pan‐proctocolectomy.21 Previous studies have addressed the prevalence and “safety” of endoscopic polypectomy for exophytic (Paris class I) adenoma‐like DALM in CUC. Rubin et al2 performed a simple polypectomy of 70 histopatholoigcally confirmed dysplastic lesions in 48 patients with no demonstrable synchronous flat dysplasia. In this initial report, there was no subsequent cancer or flat dysplastic change detected at a median follow‐up of 4.1 years (data similar to the CUC screening cohort of Engelsgjerd et al3 and Odze et al22). Furthermore, data from Connell et al23 and Negent et al,24 who performed snare polypectomy of polypoid dysplastic lesions within a colitis zone, revealed no emergence of flat dysplasia or adenocarcinoma at 2–13 and 3–11 years (median: 6 years) of follow‐up, respectively. The long‐term follow‐up series of Odze et al validate these data, in which no significant difference was reported in the prevalence of polyp formation or cancer (mean follow‐up: 82.1 months (17–156), 71.8 months (7–135) and 60.4 months (29–100) for the adenoma‐like DALM group, sporadic adenoma CUC group and sporadic adenoma non‐CUC control group, respectively).22 However, there are three fundamental key differences between one data and those of other authors—namely, previous exclusion of patients with flat dysplastic mucosal and carpet (LST) tumours, no routine marking of polypectomy sites, making the subsequent interpretation of post‐resection recurrence and metachronous lesional rates difficult to interpret, no routine use of chromoscopic assisted endoscopy, which has now been shown to benefit the characterisation and detection of IN in CUC,4 and variable definitions of non‐CUC “sporadic” control groups. Prevalence data relating to Paris class 0–II lesions within CUC have also never been included in these cohort studies with patients referred for pan‐proctocolectomy, owing to the lack of supportive data to justify endoluminal resection and surveillance.2
Previous published data by our group reported the anatomical mapping, histopathological characteristics and efficacy of EMR for Paris class 0–II and I lesions in a population, assuming a high overall lifetime risk of colorectal neoplasia (entry criteria being previous HGD adenoma and colorectal neoplasia within the past 5 years).17 We have shown for the first time that, although there was no significant difference when comparing the histopathological characteristics (ie, prevalence of LGD/HGD/invasive neoplasia) and anatomical localisation of Paris class 0–II lesions between the CUC study group and non‐CUC controls, there was a significant increase in the prevalence of Paris class 0–II lesions in the CUC group (82/155 (61%) versus 285/801 (35%); χ2 = 31.13; p<0.001). These data probably reflect the differences between the chromoscopic techniques used in these study cohorts (pan‐chromoscopy vs targeted). Previous randomised controlled data both from our group25 and from Brooker et al26 have shown a significant increase in Paris class 0–II lesional detection when using pan‐colorectal mucosal chromoscopy. Furthermore, of the 203 lesions detected during this study, 167 (82%) were detected at index screening colonoscopy, and 36 (18%) were detected by subsequent surveillance. These data are almost contrary to the post index metachronous data reported by Rubin et al,2 who detected 71% of polyps after a negative initial colonoscopy, but also represent a higher index lesional yield than those reported by Engelsgjerd et al3 and Odze et al22 (42% and 58%, respectively). Even when assuming a colonoscopic lesion, the “miss rate” of 25–30% per procedure, according to the “back to back” data of Rex et al27 and Hixson et al,28 our higher index lesional frequency and low metachronous rates at follow‐up probably reflect the augmented chromoscopic technique, and, importantly, the removal of false‐positive metachronous rates, given our mandatory tattoo marking of lesions undergoing endoluminal resection—an important limitation of all previous studies.
Recent published data suggest that using HMCC with targeted biopsies in the context of CUC surveillance significantly increases the diagnostic yield for IN fourfold compared with that obtained by conventional colonoscopy and biopsy protocols (p<0.001).4,29 However, in this study, 59 patients had IN confirmed by biopsy in non‐circumscribed lesional mucosa taken according to the BSG surveillance protocol (10 cm serial quadrantic). However, there was only one interval cancer in a patient presenting with multifocal LGD in this group. All other patients had either endoscopic apparent cancer or DALM, which would prompt mandatory pan‐proctocolectomy in any event, supporting the data of Rutter et al.6 Hence, surgical management would not have been changed in this subgroup of patients even if a further circumscribed lesion was unmasked. These data do, however, highlight the current limitations of HMCC in CUC surveillance. Chromoscopic assisted confocal endomicroscopy, as described by Kiesslich et al30 for the detection and characterisation of IN, may offer a superior endoscopic tool to HMCC in the future for IN detection in CUC surveillance. Further randomised, multicentre studies are now required to clarify this important issue.
EMR in the context of Paris class 0–II colorectal lesions has not been described in the context of CUC prior to our study. Our data show no significant difference between patients undergoing EMR in the CUC group and controls, with the primary endpoints being post‐resection recurrence, interval cancer rates and endoscopic complications (ie, procedure‐related haemorrhage/perforation). Furthermore, recurrence rates in the CUC group were lower than those described by other groups,31,32 which probably reflects the routine use of post‐EMR margin assessment,18 using HMCC in combination with post‐resection APC margin ablation for those patients receiving piecemeal dissections, or where remnant neoplastic tissue is identified at post‐resection HMCC examination.33 Low recurrence rates are essential in CUC, in which remnant adenomatous tissue has the risk of progressing to invasive carcinoma.
In conclusion, we have shown, for the first time, that flat dysplastic lesions in the context of CUC can be managed by endoluminal resection using EMR and ESD as in non‐colitic cohorts. We have verified the safety of endoluminal resection for exophytic Paris class I ALM lesions in CUC. Careful selection of lesions suitable for EMR and ESD is, however, mandatory. Training requirements in the associated adjunctive endoscopic techniques will also need to address whether routine EMR should be adopted into CUC surveillance protocols. A change in management paradigm to include EMR for the resection of flat dysplastic lesions within the context of CUC is therefore proposed by these novel data.
Acknowledgements
We thank Ian Adam, AJ Shorthouse, Paul Skinner, I Adam and S Brown for providing patients for inclusion in this study and help in the final preparation of the manuscript.
Abbreviations
ALM - adenoma‐like mass
BSG - British Society of Gastroenterology
CUC - chronic ulcerative colitis
DALM - dysplasia‐associated lesion mass
EMR - endoscopic mucosal resection
ESD - endoscopic submucosal dissection
HGP - high‐grade dysplasia
HMCC - high‐magnification chromoscopic colonoscpy
IN - invasive neoplasia
LGP - low‐grade dysplasia
LST - lateral spreading tumour
Footnotes
Funding: The Smith and Nephew Research Foundation. BRET Research Foundation. Butterfield ‘Sasakawa' Foundation (UK). Mason Medical Research Foundation and the Peel Research Foundation.
Competing interests: None.
References
- 1.Eaden J A, Mayberry J F. Colorectal cancer complicating ulcerative colitis: a review. Am J Gastroenterol 2000952710–2719. [DOI] [PubMed] [Google Scholar]
- 2.Rubin P H, Friedman S, Harpaz N.et al Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology 19991171295–1300. [DOI] [PubMed] [Google Scholar]
- 3.Engelsgjerd M, Farraye F A, Odze R D. Polypectomy may be adequate treatment for adenoma‐like dysplastic lesions in chronic ulcerative colitis. Gastroenterology 19991171288–1294. [DOI] [PubMed] [Google Scholar]
- 4.Hurlstone D P, Sanders D S, Lobo A J.et al Indigo carmine‐assisted high‐magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy 2005371186–1192. [DOI] [PubMed] [Google Scholar]
- 5.Kiesslich R, Fritsch J, Holtmann M.et al Methylene blue‐aided chromoendoscopy for the detection of intraepitheial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003124880–888. [DOI] [PubMed] [Google Scholar]
- 6.Rutter M D, Saunders B P, Schofield G.et al Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 200453256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paris Workshop Participants The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach and colon. Gastrointest Endosc 200258S3–43. [DOI] [PubMed] [Google Scholar]
- 8.Kudo S, Rubio C A, Teixeira C R.et al Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 200133367–373. [DOI] [PubMed] [Google Scholar]
- 9.Hurlstone D P, Cross S S, Shorthouse A J.et al The efficacy of high‐magnification‐chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut 200453284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Tanaka S, Haruma K.et al Pit pattern diagnosis of early colorectal carcinoma by magnifying colonoscopy: clinical and histological implications. Int J Oncol 200016927–934. [DOI] [PubMed] [Google Scholar]
- 11.Kudo S, Kashida H, Nakajima T.et al Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg 199721694–701. [DOI] [PubMed] [Google Scholar]
- 12.Carter M J, Lobo A J, Travis S P. Guidelines for the management of inflammatory bowel disease in adults. Gut 200453(Suppl 5)V1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlemper R J, Riddell R H, Kato Y.et al The Vienna classification of gastrointestinal neoplasia. Gut 200047251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higaki S, Hashimoto S, Harada K.et al Long‐term follow‐up of large flat colorectal tumors resected endoscopically. Endoscopy 200335845–849. [DOI] [PubMed] [Google Scholar]
- 15.Hurlstone D P, Cross S S, Adam I.et al An evaluation of colorectal endoscopic mucosal resection using high‐magnification chromoscopic colonoscopy: a prospective study of 1000 colonoscopies. Endoscopy 200436491–498. [DOI] [PubMed] [Google Scholar]
- 16.Hurlstone D P, Cross S S, Adam I.et al Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut 200453284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurlstone D P, Cross S S, Adam I.et al A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the UK. Am J Gastroenterol 2003982543–2549. [DOI] [PubMed] [Google Scholar]
- 18.Hurlstone D P, Cross S S, Brown S.et al A prospective evaluation of high‐magnification chromoscopic colonoscopy in predicting completeness of EMR. Gastrointest Endosc 200459642–650. [DOI] [PubMed] [Google Scholar]
- 19.Blackstone M O, Riddell R H, Rogers B H.et al Dysplasia‐associated lesion or mass (DALM) detected by colonoscopy in long‐standing ulcerative colitis: an indication for colectomy. Gastroenterology 198180366–374. [PubMed] [Google Scholar]
- 20.Hurlstone D P, Fujii T, Lobo A J. Early detection of colorectal cancer using high‐magnification chromoscopic colonoscopy. Br J Surg 200289272–282. [DOI] [PubMed] [Google Scholar]
- 21.Connell W R, Talbot I C, Harpaz N.et al Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis. Gut 1994351419–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odze R D, Farraye F A, Hecht J L.et al Long‐term follow‐up after polypectomy treatment for adenoma‐like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 20042534–541. [DOI] [PubMed] [Google Scholar]
- 23.Connell W R, Lennard‐Jones J E, Williams C B.et al Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994107934–944. [DOI] [PubMed] [Google Scholar]
- 24.Negent F W, Haggitt R C, Gilpin P A. Cancer surveillance in ulcerative colitis. Gastroenterology 19911001241–1248. [PubMed] [Google Scholar]
- 25.Hurlstone D P, Cross S S, Slater R.et al Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan‐colonic versus targeted chromoscopy. Gut 200453376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooker J C, Saunders B P, Shah S G.et al Total colonic dye‐spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest Endosc 200256333–338. [DOI] [PubMed] [Google Scholar]
- 27.Rex D K, Cutler C S, Lemmel G T.et al Colonoscopic miss rates of adenomas determined by back‐to‐back colonoscopies. Gastroenterology 199711224–28. [DOI] [PubMed] [Google Scholar]
- 28.Hixson L J, Fennerty M B, Sampliner R E.et al Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990821769–1772. [DOI] [PubMed] [Google Scholar]
- 29.Hurlstone D P, McAlindon M E, Sanders D S.et al Further validation of high‐magnification chromoscopic‐colonoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2004126376–378. [DOI] [PubMed] [Google Scholar]
- 30.Kiesslich R, Burg J, Vieth M.et al Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004127706–713. [DOI] [PubMed] [Google Scholar]
- 31.Brooker J C, Saunders B P, Shah S G.et al Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomised trial and recommendations. Gastrointest Endosc 200255371–375. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad N A, Kochman M L, Long W B.et al Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc 200255390–396. [DOI] [PubMed] [Google Scholar]
- 33.Hurlstone D P, Sanders D S, Cross S S.et al Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut 2004531334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]