It has been reported that Crohn's disease initially occurs as tiny aphthoid lesions at the sites of lymphoid follicles in the gastrointestinal tract.1,2,3 The follicle‐associated epithelium (FAE) of the gut‐associated lymphoid tissues such as Peyer's patches (PPs)3,4 is a single layer of epithelial cells covering each follicle and forms a dome between the surrounding villi.3,4 Endoscopic observation of PPs in patients with Crohn's disease has rarely been performed in clinical settings.1,2,3,5,6
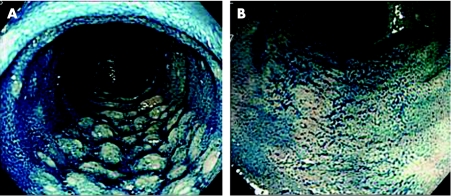
A total of seven patients with active Crohn's disease and 19 age‐ and sex‐matched healthy controls were enrolled. Chromoendoscopy was carried out with crystal violet and/or indigo carmine to identify PPs. The FAE on the domes of PPs was examined by magnifying endoscopy. The macroscopic appearance of PPs was classified into two categories, a nodular or convolute elevation pattern (E type, fig 1A) and a flat pattern (F type, fig 1B), corresponding to lymphoid follicle and lymphocyte aggregation types, respectively, as described by Fujikura et al.7 E‐type PPs are associated with definite lymphoid follicles and abundant lymphoid hyperplasia, whereas F‐type PPs consist of aggregated lymphocytes and reticulum cells, which were loosely mixed together.7 Two endoscopic biopsy specimens taken from the domes of PPs were subjected to histopathological analysis and scanning electron microscopy.3 All patients gave their written informed consent after approval by the university ethics committee.
Figure 1 Chromoendoscopic view with a magnifying videocolonoscope (Olympus CF‐240ZI, indigo carmine and crystal violet for (A) and (B), respectively). Nodular elevation of Peyer's patches (E type) and flat Peyer's patches (F type) for (A) and (B), respectively.
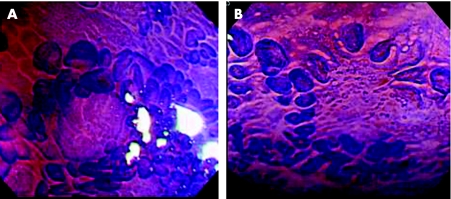
The seven patients with Crohn's disease (four men and three women) had a mean age of 26 years (range 16–42 years). Six patients had minimal lesions including small ulcers and erosions within the PPs. There were 11 E‐type and 8 F‐type PPs in the control group, whereas all the PPs in patients with Crohn's disease were classified as F type. On magnifying chromoscopy, most of the domes within PPs in controls rose into a little mound and were surrounded by dense villi (fig 2A). However, flat, distorted domes, surrounded by scattered villi (fig 2B), were identified in six patients with Crohn's disease. Repeat endoscopy in remission stage after total parental nutrition or enteral feeding with an elemental diet showed that the irregularity of domes improved in four of the six patients with Crohn's disease, accompanied by densely surrounding villi, albeit the appearance of the PPs remained of E type. A non‐caseous epithelioid granuloma was histopathologically seen in six of seven patients. On electron microscopy, M cells were seen on the domes in all cases.
Figure 2 Magnifying chromoscopic view. (A) Domes in the Peyer's patches of a control subject, surrounded by dense villi. (B) Irregularly even domes in the Peyer's patches of a patient with Crohn's disease, surrounded by scattered villi.
PPs of Crohn's disease were exclusively categorised as F type, whereas E type was predominant in the age‐ and sex‐matched controls. On observation by magnifying endoscopy, most patients with Crohn's disease had irregularly even domes surrounded by sparse villi. Initial lesions of Crohn's disease are postulated to be aphthous erosions of the domes in PPs,6 and we identified some erosions on the domes' FAE. Taken together, the F‐type PPs in Crohn's disease can reflect the irregularly affected domes with few covering villi. Thus, the FAE of patients with active Crohn's disease is likely to be more exposed to the luminal antigens and to be in closer contact with the immune system.4 Notably, the non‐caseous epithelioid granuloma was frequently identified in the biopsy specimens taken from PPs. Such minimal Crohn's disease lesions may originate from PPs and may be related to gut‐associated lymphoid tissues underlying the FAE. It has been postulated that M cells are related to the pathogenesis of Crohn's disease through their function as a portal of entry for potentially pathogenic agents.4 In fact, M cells were detected exclusively in the FAE on electron microscopy. It is difficult to obtain M cells by conventional endoscopic biopsy, because they are located on the domes but not in the villi of PPs.3,4 Therefore, magnifying endoscopy is useful to clearly recognise the FAE of the domes.
Footnotes
Competing interests: None.
References
- 1.Hizawa K, Iida M, Aoyagi K.et al The significance of colonic mucosal lymphoid hyperplasia and aphthoid ulcers in Crohn's disease. Clin Radiol 199651706–708. [DOI] [PubMed] [Google Scholar]
- 2.Fujimura Y, Kamoi K, Iida M. Pathogenesis of aphthoid ulcers in Crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut 199638724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishimoto H, Isomoto H, Shikuwa S.et al Endoscopic identification of Peyer's patches of the terminal ileum in a patient with Crohn's disease. World J Gastroenterol 2004102767–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gullberg E, Soderholm J D. Peyer's patches and M cells as potential sites of the inflammatory onset in Crohn's disease. Ann NY Acad Sci 20061072218–232. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart‐Mummery H E, Morson B C. Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut 1960187–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kruiningen H J, Ganley L M, Freda B J. The role of Peyer's patches in the age‐related incidence of Crohn's disease. J Clin Gastroenterol 199725470–475. [DOI] [PubMed] [Google Scholar]
- 7.Fujikura S, Tanaka M, Inatsuchi S.et al Ultrastructual study of Peyer's patches. J Clin Electron Microscopy 1983165–6. [Google Scholar]