Abstract
Background
Colorectal carcinoids are often described as low‐grade malignant. However, no study has compared the survival between patients with colorectal carcinoids and those with carcinomas, in a large series. In addition, no global consensus has been established on the crucial determinants of metastasis in colorectal carcinoids.
Aim
To determine the predictive factors for metastasis in colorectal carcinoids and clarify their prognosis compared with adenocarcinomas.
Methods
Data of all patients diagnosed as having colorectal carcinoids were extracted from a large nationwide database of colorectal tumours, the Multi‐Institutional Registry of Large‐Bowel Cancer in Japan, for the period from 1984 to 1998. Risk factors for lymph node (LN) metastases and distant metastases were analysed among those who were undergoing surgery, by univariate and multivariate analysis. Cancer‐specific survival was also compared between patients with colorectal carcinoids and those with adenocarcinomas registered in the same period.
Results
Among the 90 057 cases of colorectal tumours that were diagnosed, a total of 345 cases of carcinoids were identified, including 247 colorectal carcinoids of those undergoing surgery. Risk factors for LN metastasis were tumour size ⩾11 mm and lymphatic invasion, whereas those for distant metastasis were tumour size ⩾21 mm and venous invasion. Colorectal carcinoids without these risk factors exhibited no LN metastasis or distant metastasis. Cancer‐specific survival of patients with colorectal carcinoids without metastasis was better than that of those with adenocarcinomas. However, the survival was similar between carcinoids and adenocarcinomas if the tumours had LN metastasis or distant metastasis.
Conclusions
The presence of metastasis in colorectal carcinoids could lead to survival that is as poor as in adenocarcinomas. Tumours ⩽10 mm and without lymphatic invasion could be curatively treated by local resection, but others would need radical LN dissection.
The term carcinoid is synonymous with the term well‐differentiated neuroendocrine tumour,1,2 and is the most common neuroendocrine tumour of the gastrointestinal tract.3 Among such tumours, carcinoids of the colon and the rectum are grouped together in the World Health Organization (WHO) classification and are distinguished from those of the appendix or the ileum.4 In the WHO classification, colorectal carcinoids are described as “low‐grade malignant” even in the presence of metastasis.4 Furthermore, the WHO classification even defines colorectal carcinoids as “benign” if tumours are confined to the submucosa, measuring ⩽20 mm and lacking angioinvasion.1,4 However, this criterion has been recently challenged by the evidence that even such small colorectal carcinoids usually cause metastasis.5,6,7,8 In addition, it has not been clarified whether metastatic colorectal carcinoids are truly low‐grade malignant, as no study has compared the prognosis of colorectal carcinoids with that of carcinomas in a large series.
Another important discussion is the crucial determinants of metastasis in colorectal carcinoids. Indeed, numerous studies have reported various indicators of metastasis, including tumour size >10 mm or >20 mm, invasion to the muscularis propria, higher age, male gender, tumour site, histological growth pattern and mitotic rate.2,8,9,10,11 However, the results are varied, possibly due to the small number of cases available for each study. Consequently, there has been a disagreement on the therapeutic strategy for colorectal carcinoids, especially as to whether tumours between 10 and 20 mm in size need radical lymph node (LN) dissection or can be treated with local resection.2,5,7,9,10
To address these issues, the present study aims to (1) clarify the prognosis of metastatic colorectal carcinoids compared with carcinomas, (2) determine the risk factors for LN and distant metastases in colorectal carcinoids and (3) provide a strategy for the treatment of this uncommon disease, using a large nationwide database compiled in Japan over a period of 15 years.12,13
Methods
Data source for the study
The data for this study were extracted from the Multi‐Institutional Registry of Large‐Bowel Cancer in Japan, for the period from 1984 to 1998.14,15 This registry has been prospectively compiled by the Japanese Society for Cancer of the Colon and Rectum (JSCCR), which includes the departments of surgery, internal medicine, pathology and radiation at hospitals all over Japan. The member institutions are distributed throughout the country, and all patients with colorectal cancer in each institution were prospectively registered. There was no intentional inclusion or exclusion of the samples to the registry that might introduce any bias into the results. The data include detailed clinicopathological factors as defined previously by the JSCCR.16 The registry covers colon and rectal cancers, and also appendiceal tumours and some cases of ileal tumours, which were resected as ileocaecal tumours. This nationwide database covers ∼10% of all patients with colorectal cancer in Japan.14,17
Patient selection and data extraction
Data of all patients pathologically diagnosed as having carcinoid tumours during the years from 1984 to 1998 were extracted from the database along with their clinicopathological data, including gender, age, race, date and method of resection (surgery or endoscopy), tumour site, tumour size in millimeters, depth of invasion, presence of lymphatic or venous invasion, presence of LN metastasis, presence and site of distant metastasis, date of the most recent follow‐up and cancer‐specific prognosis. Histology was not reviewed again for this study due to unavailability of the specimens. Therefore, inclusion to the present study was based on the original pathological diagnosis at registration. Cancer‐specific survival, risk factors for LN metastases and distant metastases and metastatic rates were analysed among those who were undergoing surgery for carcinoids of the colon and the rectum, according to the WHO classification.4 T0 carcinoid tumours were excluded from the analysis. For comparing cancer‐specific survival and metastatic rates between patients with carcinoids and those with adenocarcinomas, data of all patients pathologically diagnosed as having well‐ and moderately differentiated adenocarcinoma in the same period were extracted from the database with these clinicopathological variables.
Dependent and independent variables in the analysis of risk factors for metastasis
The outcome of interest was LN metastases and distant metastases. LN metastasis was determined histologically in the surgical specimen. Distant metastasis was determined either radiologically before surgery, histologically in the surgical specimen or by intraoperative findings. As for independent variables, patient age (<55 and ⩾55 years), tumour size (1–10, 11–20 and ⩾21 mm) and tumour depth were evaluated as categorical variables (T2 and ⩾T3).
Statistical analysis
Statistical analysis was performed using JMP software V 6.0.0 (SAS Institute, Cary, North Carolina, USA). Survival analysis was performed using the Kaplan–Meier method, with cancer‐specific death as the outcome. Patients who died from other causes or were alive at the most recent follow‐up were treated as censored in this analysis. Therefore, all the deaths in this analysis were definitely caused by the progression of carcinoids. For the analysis of risk factors for metastasis, the univariate relationship between each clinicopathological variable and the presence of metastasis was evaluated using Pearson's χ2 test or Fischer's exact probability test, and these variables were also evaluated by multivariate logistic regression model using a Wald statistic backward stepwise selection. p Values <0.05 were considered to be significant.
Results
Site distribution of colorectal carcinoids in Japan
Among the 90 057 cases of tumours registered between 1984 and 1998, a total of 345 cases of carcinoids were identified. All cases were from the Asian population. The site distribution consisted of 3 (0.9%) ileum, 8 (2.3%) appendix, 28 (8.2%) colon and 304 (88.6%) rectum, after excluding two cases of unknown sites. Thus, carcinoids in the Japanese population exhibited a high prevalence in the rectum. Colon carcinoids occurred most often in the caecum (9/28, 32%), followed by the sigmoid colon (8/28, 28%), the transverse colon (5/28, 18%), the ascending colon (4/28, 14%) and the descending colon (2/28, 7%). The majority of rectal carcinoids developed in the lower rectum (267/304, 88%).
Clinical features of colorectal carcinoids
Table 1 shows the clinical features of 332 carcinoids in the colon and rectum. These cases demonstrated male prevalence and a mean age of 54 years. Familial history of colorectal cancer and other cancers were present in 5% and 23% of the cases, respectively. Almost 85% of the tumours were <20 mm in size and short of submucosal invasion in depth. Lymphatic and venous invasion in the surgical specimen was present in 25% and 16% of the cases, respectively. The main treatments consisted of surgery (263/327, 80%), endoscopic resection (63/327, 19%) and chemotherapy (1/327, 0.3%). Of those patients who were undergoing surgery, 11% received adjuvant chemotherapy for the primary tumours.
Table 1 Clinical features of colorectal carcinoids.
| n (%) | |
|---|---|
| Gender | |
| Male | 229 (69) |
| Female | 102 (31) |
| Mean (median), age (years) | 54 (54) |
| FH of CRC | |
| Positive | 16 (5) |
| Negative | 284 (95) |
| FH of other cancer | |
| Positive | 67 (23) |
| Negative | 228 (77) |
| Tumour site | |
| Colon | 28 (8) |
| Rectum | 304 (92) |
| Tumour size (mm) | |
| 1–10 | 186 (63) |
| 11–20 | 65 (22) |
| >21 | 43 (15) |
| Tumour depth | |
| T0 | 26 (9) |
| T1 | 237 (77) |
| T2 | 14 (5) |
| T3 | 12 (4) |
| T4 | 17 (6) |
| Lymphatic invasion | |
| Positive | 55 (25) |
| Negative | 168 (75) |
| Venous invasion | |
| Positive | 36 (16) |
| Negative | 186 (84) |
FH, familial history; CRC, colorectal cancer.
Risk factors for LN metastases and distant metastases in colorectal carcinoids
Following the above, risk factors for LN metastases and distant metastases were analysed among those who underwent surgery for carcinoids in the colon and rectum. Among the 332 patients with carcinoids in the colon and rectum, a total of 247 underwent surgery for tumours with submucosal or deeper invasion, with data on LN metastases and distant metastases available in 116 and 199 cases, respectively. The most frequent site of distant metastasis was the liver (13/14 cases, 93%), whereas one case had extra‐liver metastasis. Table 2 shows the association between clinicopathological factors and LN metastasis or distant metastasis. Age >55 years, tumour size, tumour invasion ⩾T2, lymphatic invasion and venous invasion were significantly associated with both a higher incidence of LN metastases and distant metastases. Tumour site in the colon also showed statistical correlation with distant metastasis. The variables with p<0.2 were then selected for multivariate analysis using a stepwise logistic regression model (table 3). Tumour size ⩾11 mm and lymphatic invasion were independently predictive of LN metastasis. In contrast, independent risk factors for distant metastasis were tumour size ⩾21 mm and venous invasion.
Table 2 Clinicopathological factors and lymph‐node or distant metastasis.
| LN metastasis | Distant metastasis | |||||
|---|---|---|---|---|---|---|
| Number of metastasis | Total n (%) | p Value | Number of metastasis | Total n (%) | p Value | |
| Gender | ||||||
| Male | 27 | 83 (33) | 0.581 | 9 | 138 (7) | 0.670 |
| Female | 9 | 33 (27) | 5 | 61 (8) | ||
| Age (years) | ||||||
| < = 55 | 10 | 51 (20) | 0.018 | 2 | 96 (2) | 0.008 |
| >55 | 26 | 65 (40) | 12 | 103 (12) | ||
| FH of CRC | ||||||
| Positive | 2 | 5 (40) | 0.536 | 1 | 12 (8) | 0.643 |
| Negative | 27 | 99 (27) | 9 | 173 (5) | ||
| FH of other cancer | ||||||
| Positive | 5 | 22 (23) | 0.476 | 3 | 40 (8) | 0.654 |
| Negative | 25 | 82 (31) | 8 | 143 (6) | ||
| Tumour site | ||||||
| Colon | 6 | 17 (35) | 0.681 | 6 | 21 (29) | <0.001 |
| Rectum | 30 | 99 (30) | 8 | 178 (5) | ||
| Tumour size (mm) | ||||||
| 1–10 | 3 | 43 (7) | <0.001 | 0 | 88 (0) | <0.001 |
| 11–20 | 12 | 30 (40) | 1 | 50 (2) | ||
| >21 | 19 | 33 (58) | 10 | 37 (27) | ||
| Tumour depth | ||||||
| T1 | 12 | 73 (16) | <0.001 | 2 | 144 (1) | <0.001 |
| T2–4 | 24 | 36 (67) | 12 | 41 (29) | ||
| Lymphatic invasion | ||||||
| Positive | 30 | 43 (70) | <0.001 | 11 | 52 (21) | <0.001 |
| Negative | 2 | 54 (4) | 2 | 95 (2) | ||
| Venous invasion | ||||||
| Positive | 19 | 26 (73) | <0.001 | 10 | 32 (31) | <0.001 |
| Negative | 13 | 69 (19) | 3 | 115 (3) | ||
CRC, colorectal cancer; FH, familial history; LN, lymph node.
Table 3 Multivariate analysis of risk factors for lymph‐node metastases and distant metastases.
| Lymph‐node metastasis | Distant metastasis | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | p Value | Variables | OR (95% CI) | p Value |
| Lymphatic invasion | 35.0 (8.4 to 245) | <0.001 | Venous invasion | 19 (2.9 to 377) | 0.009 |
| Tumour size >10 mm | 8.7 (1.7 to 66) | 0.0149 | Tumour size >20 mm | 19 (2.9 to 377) | 0.009 |
Proportion of LN and distant metastases in colorectal carcinoids and adenocarcinomas
Patients with well‐ and moderately differentiated adenocarcinomas constituted 91% of the overall cancers in the registry who underwent surgery for tumours with submucosal or deeper invasion (69 486/76 579). Hence, to clarify the metastatic potential of colorectal carcinoids, the actual proportion of LN metastases and distant metastases was compared between patients with colorectal carcinoids and those with well‐ and moderately differentiated adenocarcinomas who underwent surgery for tumours with submucosal or deeper invasion, according to the presence of the identified risk factors. As shown in table 4, no LN metastasis was present in carcinoids that were ⩽10 mm and without lymphatic invasion. However, the rate of LN metastasis increased to 16% in carcinoids with either one of these two risk factors, and further increased to as much as 77% in those with both of the risk factors. The risk of LN metastasis in colorectal carcinoids was not statistically lower than that of adenocarcinomas, and was even higher if the tumours had both of the risk factors. As for distant metastasis, no metastasis was present in tumours that were ⩽20 mm and without venous invasion. However, the rate of metastasis increased to 7% among tumours with either one of the two risk factors, and further increased to as much as 50% among those with both of the risk factors. Again, the risk of distant metastasis in colorectal carcinoids was not statistically lower than that of adenocarcinomas, but was even higher if the tumours had both the risk factors.
Table 4 Lymph‐node and distant metastases in colorectal carcinoids and adenocarcinomas.
| Risk factors | Carcinoids | Well‐ and moderately differentiated adenocarcinomas | p Value | ||
|---|---|---|---|---|---|
| Metastasis, n | Total, n (%) | Metastasis, n | Total, n (%) | ||
| LN metastasis | |||||
| 1–10 mm and ly– | 0 | 28 (0) | 29 | 678 (4.3) | 0.264 |
| >10 mm or ly+ | 4 | 25 (16) | 3570 | 17 968 (20) | 0.628 |
| >10 mm and ly+ | 26 | 34 (76) | 22 133 | 42 027 (53) | 0.005 |
| Distant metastasis | |||||
| 1–20 mm and v− | 0 | 87 (0) | 45 | 4274 (1.1) | 0.336 |
| >20 mm or v+ | 2 | 28 (7.1) | 2528 | 27 422 (9.2) | 0.704 |
| >20 mm and v+ | 8 | 16 (50) | 6716 | 29 242 (23) | 0.010 |
LN, lymph node; ly, lymphatic invasion; v, venous invasion; −, absent; +, present.
Cancer‐specific survival according to the extent of metastasis
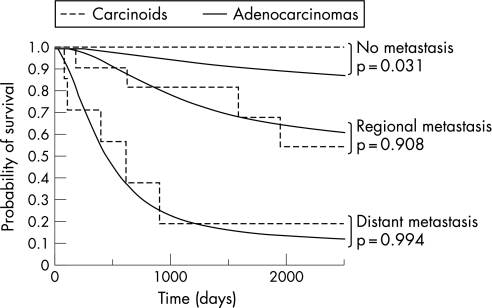
Finally, cancer‐specific survival was compared between patients with colorectal carcinoids and those with well‐ and moderately differentiated adenocarcinomas who underwent surgery for tumours with submucosal or deeper invasion, according to the extent of metastasis. Among a total of 247 patients with colorectal carcinoids and 69 486 patients with colorectal well‐ and moderately differentiated adenocarcinomas who underwent surgery for tumours with submucosal or deeper invasion, prognostic data were available for 63 and 33 875 cases, respectively. Overall median follow‐up was 68 months for patients with carcinoids and 60 months for patients with adenocarcinomas. As shown in fig 1, cancer‐specific survival of patients with colorectal carcinoids without LN or distant metastasis was significantly better than that of those with adenocarcinomas, showing 100% survival at 5 years (p = 0.031, log rank test). However, the survival rates of patients with colorectal carcinoids with regional metastasis—that is, those with LN metastasis but without distant metastasis—decreased to 81% at 3 years and 68% at 5 years, which was comparable to that of those with adenocarcinomas (p = 0.908, log rank test). Presence of distant metastasis further decreased the survival rates of patients with colorectal carcinoids to 19% at 3 years, which was also comparable to that of those with adenocarcinomas (p = 0.994, log rank test). Thus, the extent of metastasis was a crucial determinant of prognosis in both tumours, and the prognosis of colorectal carcinoids with metastasis was no better than that of those with adenocarcinomas.
Figure 1 Cancer‐specific survival of patients with colorectal carcinoids and those with adenocarcinomas. Cancer‐specific survival of patients with colorectal carcinoids without lymph node or distant metastasis was statistically better than that of those with adenocarcinomas (p = 0.031, log rank test). However, the survival rates of patients with colorectal carcinoids with regional or distant metastasis were comparable to those of patients with adenocarcinomas (p = 0.908 and p = 0.994, respectively, log rank test).
Discussion
The present study identified 345 cases of colorectal carcinoids among 90 057 colorectal cancers prospectively registered in a nationwide database in Japan. This is the largest study to date that analysed colorectal carcinoids in the Asian population. Although there have been large population‐based studies of gastrointestinal carcinoids from the US and Europe,3,18,19,20 these studies did not analyse the exact risk factors for metastasis. In contrast, the present study analysed the risk factors for metastasis among 247 cases of colorectal carcinoids undergoing surgery using detailed clinicopathological variables. This is the largest study to analyse the predictive factors for LN and distant metastases in colorectal carcinoids, and the first large‐series study that directly compared the malignant potential of colorectal carcinoids with that of adenocarcinomas.
It is generally accepted that colorectal carcinoids ⩾20 mm need radical resection for possible LN metastasis.2,7,9,10 However, the management of those that are ⩽20 mm has been controversial. Recent guidelines from the UK NeuroEndocrine Tumour group for neuroendocrine tumours describe that colorectal carcinoids ⩽10 mm could be adequately treated by endoscopic removal.10 However, there has been opposition to this strategy on the basis of the fact that LN metastasis occurs even in tumours ⩽10 mm.6,7,21 In comparison with these reports, our data revealed an incidence of LN metastasis as high as 7% in tumours ⩽10 mm. Our multivariate analysis revealed that tumour size ⩾ 10 mm and the presence of lymphatic invasion were independently predictive of LN metastasis. This result indicates that these two factors are more predictive than other evaluated variables, such as muscular invasion or higher age. Furthermore, patients without either of the two predictive factors did not exhibit LN metastasis. Our evidence suggests that colorectal carcinoids without either of the two risk factors could be curatively treated by endoscopic resection or transanal local excision without extensive LN dissection. We also emphasise that the presence of lymphatic invasion should be histologically examined in the specimens obtained by endoscopic resection or transanal local excision as this would provide useful information in deciding on considering the addition of radical surgery for LN dissection.
Distant metastasis is another point of concern in the treatment of colorectal carcinoids as it significantly worsens the prognosis.3,18 Indeed, our analysis revealed that almost 80% of patients with distant metastasis died from the disease within 3 years after surgery. Although this tumour is essentially chemoresistant,2,10,22,23,24,25 previous studies have revealed that surgical resection of the metastatic lesion could improve the survival in these patients.2,26,27,28 In particular, recent reports have indicated that surgical resection of liver metastasis should be encouraged if the extent of liver metastasis were localised.27,28,29 Therefore, early detection of metastatic lesions would be the key to achieving curative resection and prolonging the survival. In the light of the finding that tumour size >20 mm and venous invasion were the two independent risk factors for distant metastasis in the present study, patients with these risk factors would require a close follow‐up for early detection of distant metastasis.
The present study revealed that colorectal carcinoids without metastasis had better prognosis than adenocarcinomas, showing 100% 5‐year survival. Previous reports also demonstrated good prognosis in colorectal carcinoids without metastasis, showing 85–99% 5‐year survival.3,6,18 This evidence implies that colorectal carcinoids without metastasis, or at least some of them, can be truly low‐grade malignant in terms of survival. However, the present study also revealed that cancer‐specific survival of patients with colorectal carcinoids with regional or distant metastasis was comparable to that of those with adenocarcinomas. Previous studies also demonstrated poor survival in patients with metastatic colorectal carcinoids, showing 54–73% and 15–30% 5‐year survival in those with regional and distant metastases, respectively.3,6,18,30 Furthermore, the present study demonstrated that the metastatic potential of colorectal carcinoids was not lower than that of adenocarcinomas, and was even higher if the tumours had each of the two identified risk factors for metastasis. Our data are compatible with Soga's report,6 in which the metastatic rates of early‐stage rectal carcinoids were higher than those of rectal carcinomas if the tumours were >10 mm. Thus, metastatic rates of colorectal carcinoids could be as high as those of adenocarcinomas, and the survival rates of patients with colorectal carcinoids with metastasis could be as poor as those of patients with metastatic adenocarcinomas. In the WHO classification, all colorectal carcinoids are defined as low‐grade malignant, even in the presence of metastasis.1,4 This classical categorisation might be challenged by our results, at least in the case of colon and rectal carcinoids.
The current study clarified that nearly 90% of the tumours were located in the rectum. Our results were compatible with a previous report from Taiwan in which 33 of 37 (89.2%) cases of colorectal carcinoids were found to have originated from the rectum.31 Thus, over‐representation of rectal carcinoids seems to be a common feature in the Asian population. In contrast, the prevalence of colon and appendiceal carcinoids seems to be much higher among the Caucasian population.2,10,19 Such racial differences in the distribution of colorectal carcinoids suggest that race‐related genetic factors play an important role in the development of colorectal carcinoids.3
There are several notable limitations in this study. First, there are other known risk factors that were not evaluated but which could predispose to metastasis, including histological growth pattern, DNA ploidy, mitotic rate, Ki‐67 index and hormonal syndrome.1,30,32,33,34,35 These risk factors were not available in the JSCCR database. However, all variables evaluated in the present study were prospectively recorded in the database, which would have minimised accumulation of incorrect or missing data compared with retrospective research. Another potential limitation involves the accuracy of the data, as coding errors may exist. However, the validity of the JSCCR database has been well established, including metastatic rates, mortality and tumour distribution.14,15,17,36 Finally, our data include only the Asian population, and it is not clear how the data can be generalised to the rest of the world. Although the site distribution of gastrointestinal carcinoids differs between races, it has not been fully clarified whether such racial disparities similarly exist in the metastatic potential or prognosis of colorectal carcinoids. Further studies using a large international database are needed to elucidate this issue. Despite these limitations, we believe that this study reflects the actual distribution, risk factors for metastasis and survival rates of patients with colorectal carcinoids in the Asian patient population.
In conclusion, the malignant potential of colorectal carcinoids is not always lower than that of adenocarcinomas. Tumours ⩽10 mm and without lymphatic invasion could be curatively treated by endoscopic resection or transanal local excision. However, radical surgery should be considered for the dissection of regional LNs if tumours are ⩾10 mm or with lymphatic invasion. Furthermore, tumours ⩾20 mm or with venous invasion carry a high risk for distant metastasis and require a close follow‐up. These risk factors could be useful in determining the therapeutic approach.
Abbreviations
JSCCR - Japanese Society for Cancer of the Colon and Rectum
LN - lymph node
WHO - World Health Organization
Footnotes
Funding: This study was supported in part by a grant‐in‐aid for cancer research from the Ministry of Health, Labour and Welfare of Japan, and from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Competing interests: None.
References
- 1.Kloppel G, Perren A, Heitz P U. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004101413–27. [DOI] [PubMed] [Google Scholar]
- 2.Modlin I M, Kidd M, Latich I.et al Current status of gastrointestinal carcinoids. Gastroenterology 20051281717–1751. [DOI] [PubMed] [Google Scholar]
- 3.Maggard M A, O'Connell J B, Ko C Y. Updated population‐based review of carcinoid tumors. Ann Surg 2004240117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solcia E, Kloppel G, Sobin L H.et al Histological typing of endocrine tumours. WHO international histological classification of tumours. 2nd edn. Berlin: Springer, 2000
- 5.Heah S M, Eu K W, Ooi B S.et al Tumor size is irrelevant in predicting malignant potential of carcinoid tumors of the rectum. Tech Coloproctol 2001573–77. [DOI] [PubMed] [Google Scholar]
- 6.Soga J. Early‐stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 20051031587–1595. [DOI] [PubMed] [Google Scholar]
- 7.Seow‐Cheoen F, Ho J. Tiny carcinoids may be malignant. Dis Colon Rectum 199336309–310. [DOI] [PubMed] [Google Scholar]
- 8.Naunheim K S, Zeitels J, Kaplan E L.et al Rectal carcinoid tumors—treatment and prognosis. Surgery 198394670–676. [PubMed] [Google Scholar]
- 9.Shebani K O, Souba W W, Finkelstein D M.et al Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg 1999229815–21 discussion, 8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramage J K, Davies A H, Ardill J.et al Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 200554(Suppl 4)iv1–NaN16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 200519507–517. [DOI] [PubMed] [Google Scholar]
- 12.Konishi T, Watanabe T, Muto T.et al Difference in incidence of colorectal cancer between men and women in Asia. Lancet Oncol 20067104–105. [DOI] [PubMed] [Google Scholar]
- 13.Konishi T, Watanabe T, Muto T.et al The site distribution of gastrointestinal carcinoids differs between races. Gut 2006551051–1052. [PMC free article] [PubMed] [Google Scholar]
- 14.Kotake K, Honjo S, Sugihara K.et al Changes in colorectal cancer during a 20‐year period: an extended report from the multi‐institutional registry of large bowel cancer, Japan. Dis Colon Rectum 200346(10 Suppl)S32–S43. [DOI] [PubMed] [Google Scholar]
- 15.Muto T, Kotake K, Koyama Y. Colorectal cancer statistics in Japan: data from JSCCR registration, 1974–1993. Int J Clin Oncol 20016171–176. [DOI] [PubMed] [Google Scholar]
- 16.Japanese Society for Cancer of the Colon and Rectum Japanese classification of colorectal carcinoma. Tokyo: Kanehara & Co, 1997
- 17.Ajiki W, Kinoshita N, Tsukuma H.et al Cancer incidence and incidence rates in Japan in 1996: estimates based on data from 10 population‐based cancer registries. Jpn J Clin Oncol 200131410–414. [DOI] [PubMed] [Google Scholar]
- 18.Modlin I M, Lye K D, Kidd M. A 5‐decade analysis of 13,715 carcinoid tumors. Cancer 200397934–959. [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001922204–2210. [DOI] [PubMed] [Google Scholar]
- 20.Levi F, Te V C, Randimbison L.et al Epidemiology of carcinoid neoplasms in Vaud, Switzerland, 1974–97. Br J Cancer 200083952–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orloff M J. Carcinoid tumors of the rectum. Cancer 197128175–180. [DOI] [PubMed] [Google Scholar]
- 22.Oberg K, Norheim I, Lundqvist G.et al Cytotoxic treatment in patients with malignant carcinoid tumors. Response to streptozocin—alone or in combination with 5‐FU. Acta Oncol 198726429–432. [DOI] [PubMed] [Google Scholar]
- 23.Moertel C G, Hanley J A. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials 19792327–334. [PubMed] [Google Scholar]
- 24.Bajetta E, Ferrari L, Procopio G.et al Efficacy of a chemotherapy combination for the treatment of metastatic neuroendocrine tumours. Ann Oncol 200213614–621. [DOI] [PubMed] [Google Scholar]
- 25.Bajetta E, Procopio G, Ferrari L.et al Update on the treatment of neuroendocrine tumors. Expert Rev Anticancer Ther 20033631–642. [DOI] [PubMed] [Google Scholar]
- 26.Niederhuber J E, Fojo T. Treatment of metastatic disease in patients with neuroendocrine tumors. Surg Oncol Clin N Am 200615511–33, viii. [DOI] [PubMed] [Google Scholar]
- 27.Sarmiento J M, Heywood G, Rubin J.et al Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 200319729–37. [DOI] [PubMed] [Google Scholar]
- 28.Musunuru S, Chen H, Rajpal S.et al Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg 20061411000–4 discussion, 1005. [DOI] [PubMed] [Google Scholar]
- 29.Touzios J G, Kiely J M, Pitt S C.et al Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 2005241776–8383 discussion, 7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spread C, Berkel H, Jewell L.et al Colon carcinoid tumors. A population‐based study. Dis Colon Rectum 199437482–491. [DOI] [PubMed] [Google Scholar]
- 31.Weng Y J, Wang S S, Yang W G.et al Carcinoid tumors of the gastrointestinal tract in Chinese of Taiwan: an analysis of fifty cases. Zhonghua Yi Xue Za Zhi (Taipei) 199658254–258. [PubMed] [Google Scholar]
- 32.Tsioulias G, Muto T, Kubota Y.et al DNA ploidy pattern in rectal carcinoid tumors. Dis Colon Rectum 19913431–36. [DOI] [PubMed] [Google Scholar]
- 33.Burke M, Shepherd N, Mann C V. Carcinoid tumours of the rectum and anus. Br J Surg 198774358–361. [DOI] [PubMed] [Google Scholar]
- 34.Oberg K. Biochemical diagnosis of neuroendocrine GEP tumor. Yale J Biol Med 199770501–508. [PMC free article] [PubMed] [Google Scholar]
- 35.Sokmensuer C, Gedikoglu G, Uzunalimoglu B. Importance of proliferation markers in gastrointestinal carcinoid tumors: a clinicopathologic study. Hepatogastroenterology 200148720–723. [PubMed] [Google Scholar]
- 36.Takada H, Ohsawa T, Iwamoto S.et al Changing site distribution of colorectal cancer in Japan. Dis Colon Rectum 2002451249–1254. [DOI] [PubMed] [Google Scholar]