Abstract
Objective
To evaluate the associations between abdominal obesity and gastro‐oesophageal reflux disease (GORD), and their interactions with ethnicity and gender.
Design
A cross‐sectional study. Participants completed detailed symptom questionnaires and underwent a standardised examination, including anthropometric measurements.
Setting
A large integrated healthcare system.
Patients
80 110 members of the Kaiser Permanente multiphasic health check‐up cohort.
Main outcome measures
Gastro‐oesophageal reflux‐type symptoms.
Results
Recent reflux‐type symptoms were present in 11% of the population. The multivariate OR for symptoms with an abdominal diameter (adjusted for body mass index (BMI)) of ⩾26 vs <16.3 cm was 1.85 (95% CI 1.55 to 2.21) for the white population, 0.95 (95% CI 0.61 to 1.48) for the black population and 0.64 (95% CI 0.18 to 2.30) for Asians. The mean abdominal diameter was greater in men (22.0 cm, 95% CI 21.9 to 22.0) than in women (20.1 cm, 95% CI 20.0 to 20.1, p<0.01), but the risk of symptoms for any given diameter did not differ markedly by gender. The association between increasing BMI and symptoms was also much stronger among the white population than among the black population. The association between BMI and reflux‐type symptoms was partially mediated through abdominal diameter.
Conclusions
There was a consistent association between abdominal diameter (independent of BMI) and reflux‐type symptoms in the white population, but no consistent associations in the black population or Asians. The BMI association was also strongest among the white population. These findings, combined with the increased prevalence of abdominal obesity in male subjects, suggest that an increased obesity may disproportionately increase GORD‐type symptoms in the white population and in male subjects.
Gastro‐oesophageal reflux disease (GORD) is one of the most common and costly medical conditions seen in many countries.1 The identification of modifiable risk factors for GORD could potentially have a substantial public health impact.2,3,4
The incidence of GORD complications, such as oesophageal adenocarcinoma and oesophagitis, varies substantially by gender and ethnicity. GORD is strongly associated with a markedly increased risk for oesophageal adenocarcinoma,5,6 one of the most rapidly increasing cancers in several countries 7,8,9,10; however, the incidence of cancer is sixfold higher in men than in women, and is fivefold higher in Caucasians than in African Americans.11 Oesophagitis also appears to be much more common among Caucasians.12,13 There are minimal population‐based data on the prevalence of GORD, and on its risk factors stratified by gender and ethnicity.14,15 If GORD risk factors vary substantially by gender and ethnicity, this finding may partially explain the marked demographic differences in GORD complications.
Obesity may be a potential risk factor for GORD, although the results of individual studies conflict.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 In addition, simple obesity does not explain oesophageal adenocarcinoma's gender or ethnic predilections: GORD complications are most common in Caucasian men, but the prevalence of obesity is also increasing rapidly in demographic groups at a relatively low risk of oesophageal adenocarcinoma (eg, women and African Americans).33,34
The potential mechanism for the association between obesity and GORD may involve an increase in abdominal fat, resulting in increased intra‐abdominal pressure and increased oesophageal acid exposure.6,35,36,37,38 If abdominal obesity, independent of BMI, differs substantially by gender or ethnicity, it may help to explain the demographic discrepancies in GORD, but no published studies have evaluated the association between abdominal obesity and GORD in a general population.
Hence, we evaluated the associations between GORD symptoms, abdominal diameter and BMI stratified by gender and ethnicity in a large cohort, which approximates the demographics of the region's underlying general population.
Design and methods
Study design
We performed a cross‐sectional study of GORD symptoms within a large cohort.
Study population
The multiphasic cohort consists of Kaiser Permanente health‐plan members who underwent a systematic multiphasic health check‐up at facilities in San Francisco and Oakland, California; we included cohort members who were interviewed between 1964 and 1968. This cohort has been utilised for numerous risk factor studies, including evaluations of Helicobacter pylori, gastric lymphoma, gastric cancer, colorectal cancer screening, and ethnic differences in disease symptoms and outcomes.39,40,41,42,43,44,45 The Kaiser Permanente population represents the region's underlying census distributions of gender, ethnicity and socioeconomic status (except at extremes of income).46,47
Members presenting for a routine health evaluation completed a detailed, standardised questionnaire and physical examination. Detailed descriptions of study methods and validation studies have been published previously.42,43,44,45
Exposure measurements
Members underwent a standardised physical examination by trained examiners who used a written, systematic protocol with standardised instruments. The examination included anthropometric measurements (height, weight, abdominal anterior–posterior diameter and thigh diameter). The abdominal diameter was the standing anterior–posterior diameter at the iliac crest during normal breathing. The standing thigh anterior–posterior diameter was the distance from just below the left gluteal fold to the anterior thigh.
The body mass index (BMI = wt(kg)/ht(m2)) reference categories were “normal” (18.5–24.9 kg/m2); “overweight” (25–29.9 kg/m2); and “obese” (BMI ⩾30 kg/m2).48 Ethnicity was identified by the interviewer according to investigator‐specified categories, was recorded as skin colour, and is reported as “white”, “black” and “Asian”.
Quartiles used the distributions of the entire population.
Outcome measurements
Participants completed questions about symptoms within the last 6 months, including the presence or absence of “heartburn, indigestion or pain in your stomach”; the location of the discomfort; whether food intake or antacid use alleviated the symptom; symptom's relationship with position (eg, recumbency or bending over); medication use; and a history of a hiatal hernia diagnosis. For the primary analysis, GORD‐type symptoms were defined as a “yes” response to all three of the following components: the presence or absence of “heartburn, indigestion or pain in your stomach”; a location in the upper abdomen; and relief with antacid use. Additional analyses were performed using other definitions (see Supplemental analyses).
Statistical analysis
Analyses used the STATA statistical package (V.8). We evaluated the association between GORD‐type symptoms, BMI and abdominal diameter in an unconditional logistic regression; we evaluated for interaction with gender and ethnicity using cross‐product terms in the logistic regression and stratum‐specific ratios.49 We evaluated age at examination, current smoking, recent alcohol use, physical activity (total time for each daily activity), aspirin and comorbid conditions (diabetes and coronary disease) as potential confounders. In the model containing all variables, smoking and age were independently associated with GORD‐type symptoms and were included in the final model. We evaluated for confounding by conditions possibly associated with non‐reflux causes of upper gastrointestinal symptoms, such as diabetes (related to gastroparesis), coronary disease and aspirin use. They were not associated, and their inclusion did not alter the reported ORs by >10%; these variables were not included in the final model. Alcohol use was not associated with GORD‐type symptoms, but it was included in the final model, because of known clinical associations. The OR approximates the risk ratio for uncommon conditions, although it may overestimate the risk with more common conditions (such as GORD).50
The absolute abdominal diameter in centimetres (adjusted for BMI) directly contrasts different levels of abdominal diameter among persons with the same BMI. We stratified patients by BMI categories (<20, 20–22.4, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30–34.9 and >35 kg/m2), and by categories of abdominal diameter (<16.3, 16.3–17.9, 18–19.9, 20–21.9, 22–23.9, 24–25.9 and ⩾26 cm). We then calculated multivariate ORs as described above; the reference categories were a BMI of 20.0–22.4 kg/m2 and the lowest decile of abdominal diameter (<16.3 cm).
The attributable fraction calculations (ie, the proportion of GORD‐type symptoms in the population theoretically attributable to each exposure, if we assume that the statistical associations are causal) used maximum likelihood estimates from the logistic regression models that adjusted for smoking, gender, age and alcohol intake.51 The study and analyses were approved by the institutional review board.
Results
Study population
The questionnaire, BMI and abdominal measurements were available for 80 110 members. Subject characteristics are provided in table 1. The overall population was diverse in gender (56% women) and ethnicity (79% white, 13% black and 4% Asian). GORD‐type symptoms were present in 11%.
Table 1 Subject characteristics.
| n (%) | |
|---|---|
| Total | 80 110 (100) |
| Female | 44 651 (56) |
| Age at interview (years) | |
| 17–29 | 15 598 (20) |
| 30–49 | 37 047 (46) |
| 50–69 | 24 791 (31) |
| 70–89 | 2673 (3) |
| >89 | 1 (0) |
| Ethnicity | |
| White | 63 684 (79) |
| Black | 10 440 (13) |
| Asian | 3045 (4) |
| Other | 2922 (4) |
| Missing | 19 (0) |
| Current smokers (any in last year) | 34 274 (43) |
| Alcohol use in the previous year* | |
| Non‐users | 18 924 (24) |
| Up to two drinks per day | 45 472 (57) |
| More than two drinks per day | 8764 (11) |
| Body mass index (kg/m2); mean (SD) | 24.7 (4.1) |
| Underweight (<18.5) | 1971 (2) |
| Healthy weight (18.5–24.9) | 44 818 (56) |
| Overweight (25–29.9) | 26 379 (33) |
| Obese (⩾30) | 6942 (9) |
*The alcohol use category does not equal to 100%, because of missing values.
There was statistical and qualitative evidence of interaction between ethnicity, abdominal diameter categories and GORD (interaction term p values: p<0.01, white vs black subjects; p = 0.09, white vs Asian subjects; p = 0.65, black vs Asian subjects); thus, all results are stratified by ethnicity (tables 2–4).
Table 2 Gastro‐oesophageal reflux‐type symptom prevalence and distribution of body mass index and abdominal diameter (stratified by gender and ethnicity).
| White | Black | Asian | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Total in each category (n) | 35 307 | 28 377 | 6207 | 4233 | 1621 | 1424 |
| Heartburn prevalence (%) (95% CI) | 11.2 (10.8 to 11.6) | 14.3 (13.8 to 15.0) | 18.3 (17.2 to 19.5) | 15.5 (14.2 to 16.9) | 7.1 (5.7 to 8.6) | 7.8 (6.4 to 9.5) |
| BMI (kg/m2) | % | |||||
| <20 | 13.0 | 3.2 | 8.3 | 2.8 | 28.4 | 8.6 |
| 20–22.4 | 30.9 | 13.0 | 17.4 | 13.1 | 37.1 | 24.9 |
| 22.5–24.9 | 26.4 | 30.6 | 23.6 | 22.5 | 20.5 | 35.3 |
| 25–27.4 | 14.8 | 30.9 | 19.2 | 28.0 | 8.8 | 21.3 |
| 27.5–29.9 | 7.3 | 14.9 | 13.1 | 19.1 | 3.1 | 7.4 |
| 30–34.9 | 5.4 | 6.4 | 12.3 | 12.3 | 1.7 | 2.0 |
| >35 | 2.2 | 1.1 | 6.1 | 2.2 | 0.5 | 0.4 |
| Abdominal diameter (cm) | ||||||
| <16.3 | 14.8 | 4.3 | 6.8 | 3.3 | 33.6 | 11.2 |
| 16.3–17.9 | 18.1 | 7.0 | 10.3 | 7.2 | 22.7 | 15.3 |
| 18–19.9 | 22.9 | 16.2 | 17.9 | 14.9 | 22.4 | 25.0 |
| 20–21.9 | 19.6 | 24.0 | 21.3 | 21.8 | 12.3 | 23.2 |
| 22–23.9 | 11.4 | 21.5 | 16.6 | 20.8 | 5.2 | 14.7 |
| 24–25.9 | 6.1 | 14.3 | 11.3 | 15.6 | 1.7 | 7.0 |
| ⩾26 | 7.2 | 12.8 | 15.9 | 16.4 | 2.2 | 4.0 |
BMI, body mass index.
Totals may not equal 100%, because of rounding within each subcategory.
Table 3 Association between abdominal diameter and gastro‐oesophageal reflux‐type symptoms by ethnicity, with and without adjustment for body mass index.
| White | Black | Asian | |||||
|---|---|---|---|---|---|---|---|
| Total subjects* | Without adjustment for BMI | With adjustment for BMI | Without adjustment for BMI | With adjustment for BMI | Without adjustment for BMI | With adjustment for BMI | |
| Total subjects | 35 307 | 28 377 | 6207 | 4233 | 1621 | 1424 | |
| Abdominal diameter† (cm) | OR (95% CI)‡ | ||||||
| <16.3 | 7966 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 16.3–17.9 | 10 288 | 1.01 (0.89 to 1.15) | 1.01 (0.89 to 1.15) | 0.80 (0.56 to 1.12) | 0.80 (0.57 to 1.13) | 0.86 (0.52 to 1.43) | 0.83 (0.50 to 1.38) |
| 18.0–19.9 | 15 688 | 1.17 (1.05 to 1.32) | 1.12 (0.99 to 1.26) | 0.76 (0.56 to 1.04) | 0.75 (0.55 to 1.03) | 1.02 (0.64 to 1.62) | 0.91 (0.55 to 1.49) |
| 20.0–21.9 | 17 153 | 1.38 (1.23 to 1.56) | 1.22 (1.08 to 1.38) | 1.03 (0.76 to 1.39) | 0.98 (0.71 to 1.35) | 1.13 (0.69 to 1.85) | 0.80 (0.44 to 1.44) |
| 22.0–23.9 | 12 776 | 1.82 (1.61 to 2.06) | 1.47 (1.28 to 1.68) | 1.06 (0.77 to 1.46) | 0.85 (0.59 to 1.22) | 1.09 (0.58 to 2.05) | 0.62 (0.28 to 1.39) |
| 24.0–25.9 | 7970 | 1.99 (1.73 to 2.29) | 1.62 (1.37 to 1.91) | 1.21 (0.86 to 1.71) | 0.98 (0.64 to 1.50) | 1.32 (0.61 to 2.84) | 0.86 (0.30 to 2.44) |
| ⩾26 | 8269 | 2.68 (2.33 to 3.08) | 1.85 (1.55 to 2.21) | 1.67 (1.18 to 2.36) | 0.95 (0.61 to 1.48) | 1.37 (0.52 to 3.57) | 0.64 (0.18 to 2.30) |
| Test for trend§ | p<0.01 | p<0.01 | p<0.01 | p = 0.11 | p = 0.18 | p = 0.78 | |
BMI, body mass index.
*Row totals by each category of abdominal diameter.
†Reference category is the lowest decile of abdominal diameter.
‡ORs adjusted for age at examination, current smoking status, BMI and current alcohol use (see text for details).
§Test for trend across categories of abdominal diameter.
Table 4 Association between body mass index and gastro‐oesophageal reflux‐type symptoms by ethnicity and gender, not adjusted for abdominal diameter.
| White | Black | Asian | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Total subjects | 35 307 | 28 377 | 6207 | 4233 | 1621 | 1424 |
| BMI (kg/m2) | ORs (95% CI)* | |||||
| <20 | 0.94 (0.81 to 1.08) | 0.92 (0.70 to 1.22) | 0.90 (0.64 to 1.27) | 0.52 (0.23 to 1.20) | 0.94 (0.55 to 1.62) | 0.53 (0.15 to 1.91) |
| 20–22.4 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 22.5–24.9 | 1.20 (1.08 to 1.34) | 1.40 (1.21 to 1.61) | 0.91 (0.70 to 1.17) | 0.88 (0.62 to 1.24) | 0.91 (0.49 to 1.69) | 2.02 (1.09 to 3.73) |
| 25–27.4 | 1.52 (1.35 to 1.72) | 1.78 (1.55 to 2.05) | 1.17 (0.89 to 1.54) | 0.94 (0.67 to 1.32) | 0.60 (0.22 to 1.61) | 1.82 (0.91 to 3.65) |
| 27.5–29.9 | 1.64 (1.40 to 1.91) | 2.18 (1.87 to 2.55) | 0.99 (0.71 to 1.36) | 1.42 (0.99 to 2.05) | 1.35 (0.44 to 4.18) | 1.82 (0.74 to 4.48) |
| 30–34.9 | 2.31 (1.96 to 2.71) | 2.47 (2.05 to 2.96) | 1.22 (0.89 to 1.68) | 1.35 (0.90 to 2.02) | 2.57 (0.80 to 8.24) | 2.97 (0.77 to 11.37) |
| >35 | 2.80 (2.25 to 3.50) | 3.01 (2.14 to 4.23) | 1.77 (1.23 to 2.56) | 2.16 (1.09 to 4.30) | NC† | 4.57 (0.47 to 44.77) |
| Test for trend | p<0.01 | p<0.01 | p<0.01 | p<0.01 | p = 0.56 | p = 0.01 |
BMI, body mass index.
*ORs adjusted for age at examination, current smoking status and current alcohol use (see text for details).
†Not calculable due to small numbers available.
Prevalence of reflux symptoms
Reflux symptoms were relatively common for all groups. Symptoms were most common among black women (18.3%, 95% CI 17.2% to 19.5%) and least common among Asian women (7.1%, 95% CI 5.7% to 8.6%; table 2). Stratifications for age, gender, alcohol use and smoking status did not substantially alter these relationships (data not shown).
Abdominal diameter and reflux symptoms
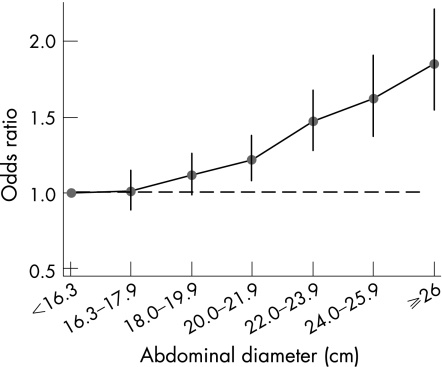
Increased abdominal diameter (adjusted for BMI) was a consistent independent risk factor for gastro‐oesophageal reflux symptoms in the white population (fig 1, table 3), but not among the black population or among Asians (table 3). There was an 85% increase in the risk of reflux symptoms in the white population between an abdominal diameter (adjusted for BMI) of <16.3 cm and an abdominal diameter of >26 cm (OR 1.85, 95% CI 1.55 to 2.21); the absolute increase in risk not adjusted for BMI was even higher (OR = 2.68, 95% CI 2.33 to 3.08). In contrast, there were no significant associations between abdominal diameter and GORD‐type symptoms for the black population or for Asians in most categories of abdominal diameter.
Figure 1 Association between reflux‐type symptoms and abdominal diameter (in cm, adjusted for BMI) among whites.
There was no statistical or consistent qualitative evidence for effect modification by gender on the association between abdominal diameter and GORD‐type symptoms (p interaction = 0.40 for all ethnicities combined; white women, OR 1.93, 95% CI 1.52 to 2.46, abdominal diameter <16.3 cm vs >26 cm; white men, OR 1.76, 95% CI 1.31 to 2.37; p value for interaction term = 0.15, ORs are adjusted for BMI).
Analyses of the abdominal diameter/thigh ratio (instead of the abdominal diameter alone) provided similar results for white subjects, but different results for black subjects. Contrasting the fourth vs first quartiles in white subjects demonstrated an increased risk of GORD‐type symptoms (white men, OR = 1.58, 95% CI 1.38 to 1.82; white women, OR = 1.45, 95% CI 1.28 to 1.64). For black subjects, the waist/thigh ratio (adjusted for BMI) was significantly associated with GORD‐type symptoms only among men (men, OR = 1.64, 95% CI 1.13 to 2.37; women, OR = 1.19, 95% CI 0.91 to 1.54), whereas the abdominal diameter alone (adjusted for BMI) was not consistently associated. For Asians, similar to the absolute abdominal diameter, there were no significant associations between the waist/thigh ratios and GORD‐type symptoms (men (fourth vs first quartile), OR = 1.18, 95% CI 0.57 to 2.44; women, OR = 1.55, 95% CI 0.75 to 3.22).
BMI and reflux symptoms
Increasing BMI was associated with reflux symptoms, but this differed by ethnicity (table 4). The risk of GORD‐type symptoms with increasing categories of BMI was much stronger for white subjects than for black subjects; the evaluation of trends among Asians was limited by the small numbers of Asians who had a BMI >30 kg/m2. Some of the BMI–GORD association was mediated through increases in abdominal diameter. Adjustment for abdominal diameter decreased the OR for GORD‐type symptoms among overweight or obese subjects (BMI ⩾25 vs <25 kg/m2; white men, OR = 1.58, 95% CI 1.47 to 1.71 vs OR = 1.39, 95% CI 1.28 to 1.52; black men, OR = 1.33, 95% CI 1.08 to 1.63 vs OR = 1.15, 95% CI 0.91 to 1.46 without and with adjustment, respectively). For Asians, BMI was not significantly associated with GORD‐type symptoms in this model.
Attributable fraction: BMI, abdominal diameter and reflux symptoms
The attributable fractions (ie, the proportion of GORD‐type symptoms in the population theoretically independently attributable to each exposure, if we assume that the associations are causal) were calculated for each ethnic group, using a logistic model containing both a BMI term and an abdominal diameter term. The attributable fractions among white subjects for a BMI⩾25 kg/m2 (vs <25 kg/m2) and an abdominal diameter ⩾18 cm (vs <18 cm) were 16.5% (95% CI 14.4 to 18.6) and 15.1% (95% CI 10.0 to 20.0), respectively; the attributable fractions among black subjects were 11.9% (95% CI 5.9 to 17.5) and 6.5% (95% CI 7.8 to 18.9), and those among Asians were 3.2% (95% CI −5.6 to 11.2) and 7.2% (95% CI −13.4 to 24.1), respectively.
Obesity and abdominal diameter
Obesity was most common in black subjects, least common in Asians, and was more common among men than among women (table 2). The mean abdominal diameter was greater in men (22.0 cm, 95% CI 21.9 to 22.0) than in women (20.1 cm, 95% CI 20.0 to 20.1; p<0.01). Men were substantially more likely to have an abdominal diameter in the third or fourth quartile than women, particularly among white subjects (fourth quartile, 32.4% of white men vs 15.8% of women, p<0.01) and Asians (14.5% of Asian men vs 5.0% of women, p<0.01); this difference was smaller among black subjects (37.5% of black men vs 31.3% of women, p<0.01). White men and black subjects were most likely to have larger abdominal diameters, and white women and Asians were the least likely (table 2). Men also had substantially higher abdominal/thigh ratios than women, and this was particularly pronounced among obese men (BMI⩾25 kg/m2). Among obese white men, 53.8% were in the fourth quartile of the abdomen/thigh ratio vs 57.1% for Asian men and 38.7% for black men. The comparable proportions for obese women were 44.4% for white women, 31.4% for Asian women and 29.5% for black women.
Supplemental analyses
The relationships between abdominal diameter and GORD‐type symptoms were similar among subgroups of patients with other symptom patterns typical for GORD‐type symptoms. For example, the OR between GORD‐type symptoms and an abdominal diameter of >26 cm in white patients was 1.85 (95% CI 1.55 to 2.21); this association had a comparable magnitude among patients in whom upper abdominal symptoms increased with bending over or lying down (OR = 1.71, 95% CI 1.28 to 2.29), or among patients who had upper abdominal symptoms that awoke them from sleep (OR = 1.93, 95% CI 1.50 to 2.47). In contrast, there were no significant associations or weaker associations between abdominal diameter and other types of abdominal pain such as gas/bloating pain (OR = 1.11, 95% CI 0.97 to 1.28), pain below the umbilicus (OR = 1.29, 95% CI 1.05 to 1.59) or abdominal pain that did not improve with antacids (1.33, 95% CI 0.98 to 1.81). The associations between GORD‐type symptoms and other potential GORD risk factors—such as age, smoking and alcohol use—were generally comparable between ethnicities, with the exception of a stronger association between smoking and GORD‐type symptoms among Asians (data not shown).
Discussion
This study has several findings: (1) there was an independent association between increasing abdominal diameter and GORD‐type symptoms in white subjects, but there was no consistent association in black subjects or Asians, despite the large size of the study; (2) there was an association between BMI and GORD‐type symptoms, particularly among white subjects; (3) GORD‐type symptoms and obesity were common among all populations; however, abdominal obesity was more common among men; (4) there were no substantial, consistent differences in the associations between GORD‐type symptoms, BMI and abdominal obesity between men and women; and (5) if the associations found are causal, the combination of BMI and abdominal diameter may account for a substantial portion of GORD‐type symptoms in the population.
This study extends the findings of previous analyses of BMI and GORD, although almost no data exist on the association between abdominal obesity and GORD.32 A recent publication from the Nurses' Health Study found an association between GORD and increasing BMI among women, but suggested that this was not influenced by the waist/hip ratio.52 In contrast, our current study determined that some of the BMI–GORD association was mediated through an increase in abdominal diameter. This difference may be due to the characteristics of the waist/hip ratio used in the Nurses' Health Study: a person with large waist and large hip measurements has a similar ratio as one with small waist and hip measurements. In contrast, analysis of the absolute abdominal diameter more directly addresses the potential for a large abdominal size alone to directly influence GORD symptoms (see the discussion of the mechanism below). Only two prior studies from the United States evaluated GORD symptoms by ethnicity: one among 496 employees at a Veterans Affairs hospital and the other among referral patients at an endoscopy unit.12,53 Both these studies suggested that black and white subjects were fairly equally likely to report heartburn, but that black subjects were less likely to have erosive oesophagitis.12,53 There are extremely little data on Asians in the United States (only 54 Asian patients in the larger of these two prior studies). Cumulatively, these studies, combined with the current analysis, suggest that GORD‐type symptoms are relatively common in all ethnic groups, but that white subjects may be more susceptible to erosive complications of GORD and may be more likely to have GORD symptoms for any incremental increase in abdominal diameter or BMI.
These findings, if causal, suggest that there may be biological differences in the mechanisms of GORD by ethnicity. Abdominal fat may cause reflux through an increase in intra‐abdominal pressure, thereby causing increased reflux.54 Although intuitive, this hypothesis is not proven and other mechanisms may exist.6,35,36,37,38 The metabolic activity of intra‐abdominal fat differs from that of peripheral fat;55 these metabolic products may influence GORD through altered gastrointestinal motility. The abdominal diameter is most strongly associated with visceral adipose tissue among persons with BMI <27 kg/m2;56 however, there may be differences in visceral adipose deposition by ethnicity. The interpretation of the BMI may also differ in different populations. The BMI calculation implies a certain relationship between height and weight, such that a regression of a function of weight on height produces a similar slope across populations; however, this relationship differs between different countries, between genders and by age, even among genetically similar groups in different geographic locales,57,58 possibly due to other differences, such as body composition (eg, weight from muscle vs fat). There are also ethnic differences in potential GORD protective factors such as gastric colonisation by the H pylori bacterium, which is less common in white subjects,59 or in motility or visceral sensitivity, which influence oesophageal acid clearance or sensation.60 It is not clear, however, whether these additional factors would be influenced by BMI or an abdominal‐fat distribution pattern.
This analysis has several strengths. First, the population consisted of a diverse patient group from a broad geographic base; hence, the results can probably be generalised to similar large populations. Second, the data were of high quality. The measurements were prospectively obtained by using a systematic protocol; the data from this cohort have been validated and used in numerous studies.39,40,41,42,43,44,45 Third, the sample size is extremely large, which allowed well‐powered evaluations of subpopulations, such as by gender and ethnicity, and the analysis of interactions. Fourth, the associations appeared robust across a variety of reflux‐type questions, and were not present for other common abdominal symptoms. Finally, the availability of comprehensive questionnaires permitted the analysis of several potential confounders.
This analysis also has several potential limitations. First, cross‐sectional studies cannot establish cause and effect, as the exposures and outcomes are measured simultaneously.50 However, if a high BMI caused GORD, we might expect the GORD to lead to weight reduction; under these circumstances, the BMI–GORD association would become weaker only if the BMI was measured at the time of interview. Second, the outcome measure, which could include both reflux and some non‐reflux upper abdominal symptoms, probably misclassified some patients with non‐reflux dyspepsia as having “reflux‐type” symptoms. We incorporated several qualifying questions that are more specific for GORD (including amelioration with antacids and relation to recumbency or bending over), but some residual misclassification is likely to remain. Third, there may have been differences in interpretation of the questions between the groups, and overlap between reflux‐type symptoms and other upper gastrointestinal symptoms. The term “heartburn”, for example, may be less well understood among Asians.12 We addressed this by using only the descriptive questions rather than medical jargon, and by evaluating several different types of questions, but residual differences may have persisted. Fourth, it is unknown whether the primary association of importance is total abdominal fat/size or only intra‐abdominal fat. Abdominal diameter is an indirect and imperfect measure of intra‐abdominal fat, and the associations may vary by ethnicity.56 Finally, observational studies are subject to confounding by other factors. Although additional analyses that adjusted for physical activity and the presence of some other comorbidities (diabetes, coronary artery disease, etc) suggested that there was little evidence of confounding by these factors (data not shown), we cannot exclude confounding by unmeasured factors (such as differences in diet) or incomplete control of confounding by measured factors.
In summary, this is the first evaluation of risk factors for GORD‐type symptoms in a large multiethnic population, and the first evaluation of abdominal size as a risk factor for GORD‐type symptoms in a large population. The results suggested that there were substantial ethnic differences in the associations between BMI, abdominal diameter and GORD‐type symptoms in our population, but there were no consistent differences by gender. Cumulatively, if the associations are causal, the data suggest that abdominal diameter in our population is an independent risk factor for GORD‐type symptoms in white subjects, that much of the observed BMI–GORD association is mediated through the abdominal diameter in white subjects but possibly not in black subjects or Asians, that there are no substantial gender differences in the role of BMI or abdominal diameter for men vs women, and that a substantial portion of the risk for GORD‐type symptoms in our population may be explained by increasing abdominal diameter and BMI. These findings suggest that recent increases in obesity, combined with the increased prevalence of abdominal obesity in men, may have disproportionately affected GORD and GORD complications (such as oesophagitis and oesophageal adenocarcinoma) in white subjects more than in black subjects or Asians, and in men more than in women. Further research is needed to evaluate whether modifications of BMI (and the resultant changes in abdominal size) can decrease GORD, and to evaluate the mechanisms through which similar anthropometric changes can have disparate impacts on different demographic groups.
Acknowledgements
The principal investigator (DAC) had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
BMI - body mass index
GORD - gastro‐oesophageal reflux disease
Footnotes
Funding: Funding was provided by the United States National Institutes of Health grants RO1 DK63616 and K08 DK02697, and a Kaiser Permanente Community Benefit Grant.
Competing interests: None.
References
- 1.Beck I T, Champion M C, Lemire S.et al The second Canadian consensus conference on the management of patients with gastroesophageal reflux disease. Can J Gastroenterol 199711(Suppl B)7B–20B. [PubMed] [Google Scholar]
- 2.Dent J, El‐Serag H B, Wallander M A.et al Epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 200554710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberger N J. Update in gastroenterology. Ann Intern Med 1998129309–316. [DOI] [PubMed] [Google Scholar]
- 4.Kulig M, Nocon M, Vieth M.et al Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGORD study. J Clin Epidemiol 200457580–589. [DOI] [PubMed] [Google Scholar]
- 5.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999130883–890. [DOI] [PubMed] [Google Scholar]
- 6.Reid B J, Barrett M T, Galipeau P C.et al Barrett's esophagus: ordering the events that lead to cancer. Eur J Cancer Prev 19965(Suppl 2)57–65. [DOI] [PubMed] [Google Scholar]
- 7.Blot W J, Devesa S S, Fraumeni J F., Jr Continuing climb in rates of esophageal adenocarcinoma: an update [letter]. JAMA 19932701320. [PubMed] [Google Scholar]
- 8.Blot W J, Devesa S S, Kneller R W.et al Rising incidence of adenocarcinoma of the esophagus and gastric cardia [see comments]. JAMA 19912651287–1289. [PubMed] [Google Scholar]
- 9.Blot W J, McLaughlin J K. The changing epidemiology of esophageal cancer. Semin Oncol 1999262–8. [PubMed] [Google Scholar]
- 10.Kubo A, Corley D A. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer 2002952096–2102. [DOI] [PubMed] [Google Scholar]
- 11.Kubo A, Corley D A. Marked multi‐ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol 200499582–588. [DOI] [PubMed] [Google Scholar]
- 12.Spechler S J, Jain S K, Tendler D A.et al Racial differences in the frequency of symptoms and complications of gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 2002161795–1800. [DOI] [PubMed] [Google Scholar]
- 13.El‐Serag H B, Johanson J F. Risk factors for the severity of erosive esophagitis in Helicobacter pylori‐negative patients with gastroesophageal reflux disease. Scand J Gastroenterol 200237899–904. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed I, Nightingale P, Trudgill N J. Risk factors for gastro‐oesophageal reflux disease symptoms: a community study. Aliment Pharmacol Ther 200521821–827. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan R, Tutuian R, Schoenfeld P.et al Profile of GORD in the adult population of a northeast urban community. J Clin Gastroenterol 200438651–657. [DOI] [PubMed] [Google Scholar]
- 16.Kotzan J, Wade W, Yu H H. Assessing NSAID prescription use as a predisposing factor for gastroesophageal reflux disease in a Medicaid population. Pharm Res 2001181367–1372. [DOI] [PubMed] [Google Scholar]
- 17.Ruigomez A, Garcia Rodriguez L A, Wallander M A.et al Natural history of gastro‐oesophageal reflux disease diagnosed in general practice. Aliment Pharmacol Ther 200420751–760. [DOI] [PubMed] [Google Scholar]
- 18.Talley N J, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol 2004991807–1814. [DOI] [PubMed] [Google Scholar]
- 19.El‐Serag H B, Graham D Y, Satia J A.et al Obesity is an independent risk factor for GORD symptoms and erosive esophagitis. Am J Gastroenterol 20051001243–1250. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed I, Cherkas L F, Riley S A.et al Genetic influences in gastro‐oesophageal reflux disease: a twin study. Gut 2003521085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke GR I I I, Talley N J, Fett S L.et al Risk factors associated with symptoms of gastroesophageal reflux. Am J Med 1999106642–649. [DOI] [PubMed] [Google Scholar]
- 22.Murray L, Johnston B, Lane A.et al Relationship between body mass and gastro‐oesophageal reflux symptoms: The Bristol Helicobacter Project. Int J Epidemiol 200332645–650. [DOI] [PubMed] [Google Scholar]
- 23.Nandurkar S, Locke GR I I I, Fett S.et al Relationship between body mass index, diet, exercise and gastro‐oesophageal reflux symptoms in a community. Aliment Pharmacol Ther 200420497–505. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M, Johnsen R, Ye W.et al Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA 200329066–72. [DOI] [PubMed] [Google Scholar]
- 25.Stene‐Larsen G, Weberg R, Froyshov Larsen I.et al Relationship of overweight to hiatus hernia and reflux oesophagitis. Scand J Gastroenterol 198823427–432. [DOI] [PubMed] [Google Scholar]
- 26.Wang J H, Luo J Y, Dong L.et al Epidemiology of gastroesophageal reflux disease: a general population‐based study in Xian of Northwest China. World J Gastroenterol 2004101647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson L J, Ma W, Hirschowitz B I. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol 1999942840–2844. [DOI] [PubMed] [Google Scholar]
- 28.Lagergren J, Bergstrom R, Nyren O. No relation between body mass and gastro‐oesophageal reflux symptoms in a Swedish population based study. Gut 20004726–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson M, Lundegardh G, Carling L.et al Body mass and reflux oesophagitis: an oestrogen‐dependent association? Scand J Gastroenterol 200237626–630. [DOI] [PubMed] [Google Scholar]
- 30.Wu A H, Tseng C C, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer 200398940–948. [DOI] [PubMed] [Google Scholar]
- 31.Incarbone R, Bonavina L, Szachnowicz S.et al Rising incidence of esophageal adenocarcinoma in Western countries: is it possible to identify a population at risk? Dis Esophagus 200013275–278. [DOI] [PubMed] [Google Scholar]
- 32.Hampel H, Abraham N S, El‐Serag H B. Meta‐analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 2005143199–211. [DOI] [PubMed] [Google Scholar]
- 33.Rosner B, Prineas R, Loggie J.et al Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr 1998132211–222. [DOI] [PubMed] [Google Scholar]
- 34.Devesa S S, Blot W J, Fraumeni J F., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998832049–2053. [PubMed] [Google Scholar]
- 35.Wajed S A, Streets C G, Bremner C G.et al Elevated body mass disrupts the barrier to gastroesophageal reflux; discussion 1018–9. Arch Surg 20011361014–1018. [DOI] [PubMed] [Google Scholar]
- 36.Ruhl C E, Everhart J E. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: the NHANES I Epidemiologic Followup Study. First National Health and Nutrition Examination Survey. Ann Epidemiol 19999424–435. [DOI] [PubMed] [Google Scholar]
- 37.Lambert D M, Marceau S, Forse R A. Intra‐abdominal pressure in the morbidly obese. Obes Surg 2005151225–1232. [DOI] [PubMed] [Google Scholar]
- 38.Galmiche J P, Janssens J. The pathophysiology of gastro‐oesophageal reflux disease: an overview. Scand J Gastroenterol Suppl 19952117–18. [DOI] [PubMed] [Google Scholar]
- 39.Selby J V, Friedman G D, Quesenberry C P., Jret al A case–control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992326653–657. [DOI] [PubMed] [Google Scholar]
- 40.Parsonnet J, Friedman G D, Vandersteen D P.et al Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 19913251127–1131. [DOI] [PubMed] [Google Scholar]
- 41.Parsonnet J, Hansen S, Rodriguez L.et al Helicobacter pylori infection and gastric lymphoma. N Engl J Med 19943301267–1271. [DOI] [PubMed] [Google Scholar]
- 42.Alexander M, Grumbach K, Selby J.et al Hospitalization for congestive heart failure. Explaining racial differences. JAMA 19952741037–1042. [PubMed] [Google Scholar]
- 43.Collen M F, Cutler J L, Siegelaub A B.et al Reliability of a self‐administered medical questionnaire. Arch Intern Med 1969123664–681. [PubMed] [Google Scholar]
- 44.Cutler J L, Ramcharan S, Feldman R.et al Multiphasic checkup evaluation study. 1. Methods and population. Prev Med 19732197–206. [DOI] [PubMed] [Google Scholar]
- 45.Selby J V, Friedman G D, Collen M F. Sigmoidoscopy and mortality from colorectal cancer: the Kaiser Permanente Multiphasic Evaluation Study. J Clin Epidemiol 198841427–434. [DOI] [PubMed] [Google Scholar]
- 46.Hiatt R A, Friedman G D. The frequency of kidney and urinary tract diseases in a defined population. Kidney Int 19822263–68. [DOI] [PubMed] [Google Scholar]
- 47.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census‐based methodology. Am J Public Health 199282703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Center for Disease Control and Prevention BMI—Body Mass Index: BMI for adults. Atlanta, GA: CDC, 2005
- 49.Hosmer D W, Lemeshow S.Applied logistic regression. 2nd edn. New York: John Wiley & Sons, 2000
- 50.Rothman K J, Greenland S.Modern epidemiology. 2nd edn. Philadelphia: Lippincott‐Raven, 1998
- 51.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 199349865–872. [PubMed] [Google Scholar]
- 52.Jacobson B C, Somers S C, Fuchs C S.et al Body‐mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 20063542340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El‐Serag H B, Petersen N J, Carter J.et al Gastroesophageal reflux among different racial groups in the United States. Gastroenterology 20041261692–1699. [DOI] [PubMed] [Google Scholar]
- 54.El‐Serag H B, Tran T, Richardson P.et al Anthropometric correlates of intragastric pressure. Scand J Gastroenterol 200641887–891. [DOI] [PubMed] [Google Scholar]
- 55.Weight Control and Physical Activity IARC handbooks of cancer prevention. 6 vols. Lyon: International Agency for Cancer Research, 2002
- 56.Schoen R E, Thaete F L, Sankey S S.et al Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord 199822338–342. [DOI] [PubMed] [Google Scholar]
- 57.Long A E, Prewitt T E, Kaufman J S.et al Weight–height relationships among eight populations of West African origin: the case against constant BMI standards. Int J Obes Relat Metab Disord 199822842–846. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez J R, Heo M, Heymsfield S B.et al Is percentage body fat differentially related to body mass index in Hispanic Americans, African Americans, and European Americans? Am J Clin Nutr 20037771–75. [DOI] [PubMed] [Google Scholar]
- 59.Parsonnet J, Replogle M, Yang S.et al Seroprevalence of CagA‐positive strains among Helicobacter pylori‐infected, healthy young adults. J Infect Dis 19971751240–1242. [DOI] [PubMed] [Google Scholar]
- 60.Wigington W C, Johnson W D, Minocha A. Epidemiology of irritable bowel syndrome among African Americans as compared with whites: a population‐based study. Clin Gastroenterol Hepatol 20053647–653. [DOI] [PubMed] [Google Scholar]