Abstract
Background
Infiltration of inflammatory cells into the colon plays an important role in the onset and course of inflammatory bowel disease. G‐protein‐coupled receptor kinase 6 (GRK6) is an intracellular kinase that regulates the sensitivity of certain G‐protein‐coupled receptors, including those involved in the migration of inflammatory cells. Therefore, it is hypothesised that GRK6 plays a role in determining the course of inflammation.
Aim
To analyse the role of GRK6 in the course of dextran sodium sulphate (DSS)‐induced colitis.
Methods
Colitis was induced by administering 1% DSS in drinking water to GRK6−/−, GRK6+/− and wild‐type (WT) mice for 6 days. The severity of colitis was assessed on the basis of clinical signs, colon length and histology. Moreover, keratinocyte‐derived chemokine (KC) levels, granulocyte infiltration, interleukin 1β (IL1β), CD4, CD8 and forkhead box protein P3 (FoxP3) expression in the colon were determined. In addition, regulatory T cell function in WT and GRK6−/− mice was analysed. The chemotactic response of granulocytes to colon culture supernatants was assessed using a transendothelial migration assay.
Results
The severity of colitis was increased in GRK6−/− and GRK6+/− mice and was accompanied by increased KC levels and increased granulocyte infiltration. Moreover, the chemotactic response of GRK6−/− granulocytes to supernatants of colon cultures was enhanced. Interestingly, the WT mice completely recovered from colitis, whereas the GRK6−/− and GRK6+/− mice developed chronic colitis, which was accompanied by increased IL1β and CD4 expression and decreased FoxP3 expression. Moreover, regulatory T cell function was impaired in the GRK6−/− mice.
Conclusions
The intracellular level of GRK6 is an important factor in determining the onset, severity and chronicity of DSS‐induced colitis.
Ulcerative colitis is a chronic, relapsing–remitting gastrointestinal disease of unknown origin. It is associated with inflammation of the superficial layer of the colon mucosa.1 A widely used animal model for the disease is dextran sodium sulphate (DSS)‐induced colitis. The histological phenotype of the acute phase of DSS‐induced colitis is characterised by epithelial cell lesions and acute inflammation, mainly consisting of infiltrating granulocytes and macrophages.2,3 Dieleman et al4 have reported that lymphocytes are not necessary for the acute inflammatory phase of DSS‐induced colitis. However, mice lacking T cells do not fully recover from colitis, suggesting that these cells play an important role in the remission of DSS‐induced colitis.4,5 Exposure of animals to several cycles of DSS results in the development of chronic colitis that is associated with infiltrates of CD4 lymphocytes and B cells.6,7
Chemokines and chemokine (CC motif) receptors (CCRs) play an important role in colitis, and mice deficient in CCR2, CCR5 or CCR6 are protected from DSS‐induced colitis.8,9,10 Chemokines act through G‐protein‐coupled receptors (GPCRs), whose responsiveness can be attenuated by G‐protein‐coupled receptor kinases (GRKs), in a process called homologous receptor desensitisation. GRKs are capable of phosphorylating agonist‐activated GPCRs, thereby inducing rapid uncoupling of the receptor from the G protein. GRK‐mediated phosphorylation of receptors also promotes binding of arrestins, which prevents further activation by promoting receptor internalisation.11,12,13 The intracellular level of GRKs is a crucial factor in determining the extent of receptor desensitisation and internalisation and consequently the level of GPCR sensitivity.14,15
The GRK family consists of seven members, GRK1–7. GRK2, 3 and 6 are highly expressed in the immune system.16,17 Reduction in GRK6 protein level leads to increased sensitivity of several receptors involved in acute inflammation, such as chemokine (CXC motif) receptor 4 and the leucotriene B4 (LTB4) receptor.18,19,20 Moreover, acute ear inflammation induced by topical administration of arachidonic acid, a precursor of LTB4, is increased in GRK6−/− mice. The increased ear swelling was associated with increased granulocyte infiltration in the ear.18 Because chemotaxis is crucial for the process of inflammation, and because the absence of GRK6 has a major effect on in vitro chemotactic responses of leucocytes, we investigated the role of GRK6 in a clinically relevant model of inflammatory disease, DSS‐induced colitis.
Materials and methods
Animals
GRK6‐deficient mice from a mixed C57Bl/6×SVJ/129 background20 were back‐crossed for eight generations to C57BL/6. We used 12–14‐week‐old male offspring of GRK6+/−×GRK6+/− mice in all experiments. Animals were genotyped by PCR and housed in the Central Animal Facility, Utrecht University, Utrecht, The Netherlands. Experiments were performed in accordance with international guidelines and approved by the experimental animal committee of the University Medical Center, Utrecht. Colitis was induced by addition of 1% DSS (molecular weight 40 000; ICN Biomedicals, Eschwege, Germany) to drinking water for 6 days. From day 7 onwards, the animals received normal drinking water. DSS consumption, body weight, stool consistency and faecal blood loss were recorded daily. Faecal blood loss was assessed using the Hemoccult test (Beckman Coulter, Krefeld‐Fischeln, Germany). The Disease Activity Index (DAI)2 was calculated as table 1 describes.
Table 1 Scoring of the Disease Activity Index (DAI).
| Score | Weight loss (%) | Stool consistency | Faecal blood |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stool | Hemoccult positive |
| 3 | 10–15 | ||
| 4 | 15–20 | Diarrhoea | Gross bleeding |
The DAI is the combined scores for weight loss, stool consistency and bleeding, leading to a maximum DAI of 12.
Normal stool, firm and well‐formed pellets; loose stool, pasty and semi‐formed stool that does not stick to the anus; diarrhoea, liquid stool that sticks to the anus.
At day 6, 16 or 70, mice were killed. After the colon length was measured, the colon was flushed with 1 ml of phosphate‐buffered saline for determination of myeloperoxidase (MPO) and eosinophil peroxidase (EPO). One half of the colon was fixed in 4% paraformaldehyde, embedded in paraffin wax and stained with H&E for histological examination. The other half was frozen in liquid nitrogen and used for cytokine measurements and RNA extraction.
Cytokine determination
Tissue was sonicated (3×5s) in phosphate‐buffered saline (100 mg tissue/ml) and centrifuged at 13 000 g for 15 min at 4°C. Keratinocyte‐derived chemokine (KC) content was determined by ELISA (R&D Systems, San Diego, California, USA). 100% = 12.31 pg KC/mg protein.
Myeloperoxidase/eosinophil peroxidase
MPO and EPO activities were determined as described previously.21 Samples were centrifuged (15 min, 13 000 g, 4°C), and supernatants were diluted 1:5 in 10 mM HEPES, pH 8.0, containing 0.22% EPO (Sigma, St Louis, Massachusetts, USA) or 10 mM citrate (Merck, Darmstadt, Germany), pH 5.0 with 0.22% MPO (Sigma). The reaction buffer for the EPO assay consisted of 3 mM o‐phenylenediamine (Sigma) and 8.8 mM H2O2 (Merck) in 10 mM HEPES, pH 8.0. The reaction buffer for the MPO assay consisted of 3 mM 3′,3′,4′,4′‐tetramethylbenzidine (Sigma), 120 μM resorcinol (Aldrich, St Louis, Massachusetts, USA) and 2.2 mM H2O2 in distilled water. Samples were diluted 1:1 in reaction buffer. The reaction was stopped with 150 μl of 2 M H2SO4 and absorbance was read at 490 nm for the EPO assay and at 450 nm for the MPO assay. Standards were prepared using isolated human neutrophils or eosinophils.
Colon culture and chemotactic assay
After 3 days of treatment with 1% DSS, colon sections (10 mm length) of wild‐type (WT) mice were incubated for 24 h at 37°C in medium.22
Transendothelial migration assays were performed as described previously.20 Briefly, 105 Ea.hy926 endothelial cells were plated onto 24‐well transwell inserts (polycarbonate filters, pore size 5 μm, Corning, New York, USA) and incubated for 3 days. Monolayer integrity was determined by assessing the diffusion of [14C] mannitol (Amersham, Roosendaal, The Netherlands) over the insert. Transwells were used when the diffusion of [14C] mannitol was <35%. The medium at the bottom was replaced with either 600 μl of culture medium or supernatant from the colon culture. WT or GRK6−/− bone‐marrow‐derived polymorpho nuclear cells were isolated and 5×105 cells were added to the top chamber. After 4 h of incubation at 37°C, the number of cells in the lower chamber was determined by flow cytometry (FACSCalibur, PharMingen–Becton Dickinson, San Diego, California, USA).
Real‐time reverse transcriptase PCR
Total RNA was extracted using Trizol (Invitrogen, Breda, The Netherlands). RNA concentration was determined spectrophotometrically and quality was assessed after agarose electrophoresis. cDNA was synthesised with the Superscript RNase H− Reverse Transcriptase Kit (Invitrogen) using 2.5 μM random hexamers (Invitrogen). Real‐time PCR was performed using CyberGreen probe with the I‐cylcer IQ5 (Bio‐Rad, Alphen a/d Rijn, The Netherlands).
Primer pairs used are as follows: CD8: sense 5′‐GCTCAGTCATCAGCAACTCG‐3′, antisense 5′‐GCCGACAATCTTCTGGTCTC‐3′; CD4: sense 5′‐GCTCAAGGAGACCACCATGTGT‐3′, antisense 5′‐GCGAAGGCGAACCTCCTC‐3′; IL1β: sense 5′‐CAACCAACAAGTGATATTCTCCATG‐3′, antisense 5′‐GATCCACACTCTCCAGCTGCA‐3′. Data were normalised using 18s rRNA expression (sense 5′‐GTAACCCGTTGAACCCCATT‐3′, antisense 5′‐CCATCCAATCGGTAGTAGCG‐3′).
Western blot
Lysates from colons of WT and GRK6−/− mice were obtained by sonication in ice‐cold radioimmunoprecipitation assay buffer (50 mM Tris, 1% Nonidet‐P40, 150 mM NaCl, 0.5 mM EDTA, 1% sodium dodecyl sulphate, 5 mg/ml sodium deoxycholate) supplemented with tissue protease inhibitors (Sigma) and 1 mM phenylmethylsulphonyl fluoride. A total of 30 μg protein was separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Hybond‐C, Amersham) by electroblotting. Blots were stained with mouse anti‐FoxP3 (1:1000; Abcam, Cambridge, UK) and goat anti‐β‐actin (1:5000; Santa Cruz Biotechnology, Santa Cruz, California, USA). Immunoreactivity was detected by enhanced chemiluminescence (Amersham). Band density was determined using a GS‐700 Imaging Densitometer (Bio‐Rad).
Cell subset isolation and proliferation assay
CD4+CD25− and CD4+CD25+ T cells were isolated from the spleen by using the CD4+CD25+ regulatory T cell (Treg) purification kit (Miltenyi Biotec, Auburn, California, USA) according to the manufacturer's protocol. Suppression assays were conducted for 72 h with 1×105 CD4+CD25− T cells and 2×105 CD4+CD25+ Treg of either WT or GRK6−/− mice. Flat‐bottom 96‐well plates were coated with 100 μl of 1 μg/ml α−CD3 solution overnight at room temperature. Cells were pulsed during the final 18 h of culture with [3H]thymidine (Amersham).
Statistical analysis
Data are expressed as means (SEM). Data were analysed by analysis of variance (ANOVA) followed by Tukey analysis, two‐way ANOVA followed by Bonferroni analysis or independent samples t test, using SPSS V.12. DAI scores were analysed with repeated‐measures ANOVA. Frequency distributions were analysed using Fisher's exact test. A p value <0.05 was considered significant.
Results
Increased severity and advanced onset of colitis in GRK6−/− mice
We induced DSS colitis by adding 1% DSS to the drinking water of GRK6−/−, GRK6+/− and WT mice for 6 days. Weight, stool consistency and blood loss were scored to calculate the DAI, as table 1 describes. Interestingly, GRK6−/− and GRK6+/− mice showed an advanced onset of DSS‐induced colitis compared with WT animals (table 2).
Table 2 Onset of colitis.
| Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|
| Wild type | 0/25 | 4/25 | 13/25 | 15/25 |
| GRK6+/− | 4/19* | 11/19** | 18/19** | 19/19*** |
| GRK6−/− | 9/26** | 19/26*** | 22/26* | 25/26** |
GRK6, G‐protein‐coupled receptor kinase.
Values are number of animals with loose stool or diarrhoea/total number of animals.
Data were analysed by Fisher's exact test.
*p<0.05, **p<0.01, ***p<0.001 versus wild‐type mice.
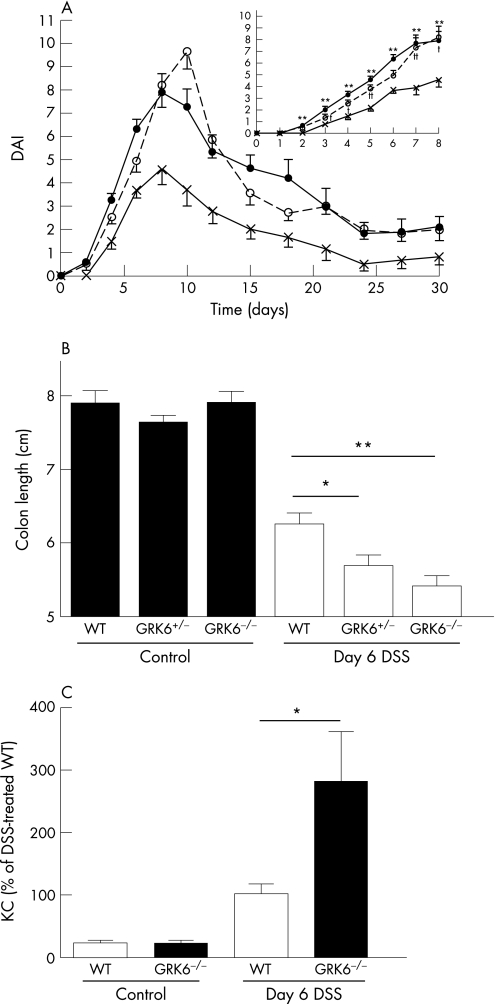
Moreover, the severity of clinical symptoms was significantly increased in both GRK6−/− and GRK6+/− mice compared with WT mice (fig 1A). DSS intake did not differ between the animals (data not shown). At day 6, the WT mice had a 20% reduction in colon length. The reduction in colon length was significantly more pronounced in the GRK6+/− (fig 1B; p<0.05) and GRK6−/− animals (fig 1B; p<0.01).
Figure 1 Course of dextran sodium sulphate (DSS)‐induced colitis. Colitis was induced by giving animals 1% DSS in drinking water for 6 days, followed by normal drinking water. (A) Mean Disease Activity Index (DAI) till day 30, based on changes in body weight, stool consistency and blood loss through faeces. Wild type (WT; cross; n = 12–32), G‐protein‐coupled receptor kinase 6 (GRK6+/−; closed circle; n = 7–19) and GRK6−/− (open circle; n = 10–33) mice. Inset shows daily DAI of colitis till day 8. (B) Colon length determined on day 6 (n = 4–12 per group). DSS‐treated animals significantly differed from healthy controls (p<0.001). (C) Concentration of keratinocyte‐derived chemokines (KCs) in homogenates of colon obtained at day 6 (n = 7–11 per group). Data are presented as means (SEM). *p<0.05, **p<0.01 GRK6−/− vs WT. †p<0.05, ††p<0.01 GRK6+/− vs WT.
To investigate whether the increased severity of colitis in the GRK6−/− animals at day 6 was accompanied by increased production of inflammatory mediators, we measured the KC concentration in colonic tissue. Colons of DSS‐treated GRK6−/− mice contained significantly more KC than colons of WT mice (fig 1C; p<0.05).
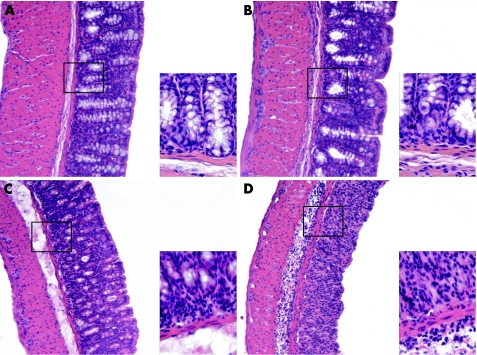
Histological examination showed increased signs of colitis in the colons of GRK6−/− mice; cellular infiltration, mainly consisting of granulocytes confined to the submucosa and sometimes extending into the lamina propria, was more pronounced in the GRK6−/− mice than in the WT mice. Moreover, crypt loss and erosions were more extensive in the GRK6−/− mice than in the WT mice (fig 2).
Figure 2 Histological sections of inflamed colons at day 6. Representative tissue sections of (A) wild‐type mice (WT), (B) G protein‐coupled receptor kinase (GRK6−/−) mice without colitis, (C) WT mice; and (D) GRK6−/− mice with colitis at day 6. Overview at 100× magnification. Insets at 500× magnification. The colons of GRK6−/− mice showed increased cellular infiltration, mainly confined to the submucosa, more extensive crypt loss and increased epithelial cell layer erosions compared with colons of WT mice.
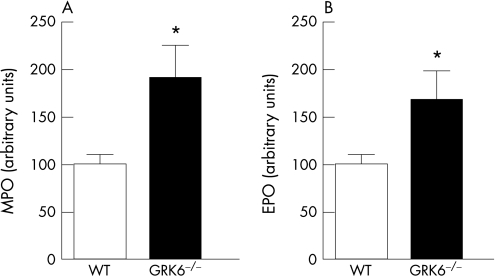
Infiltration of neutrophils and eosinophils in the colon was quantified by determining MPO and EPO activities. At day 6, both MPO and EPO levels were significantly higher in GRK6−/− mice than in WT mice (fig 3; p<0.05). In all naive animals, MPO or EPO in the colon was below detection levels. Increased infiltration of granulocytes into the colon of GRK6−/− mice was not associated with increased numbers of granulocytes in blood (data not shown).19
Figure 3 Myeloperoxidase (MPO) and eosinophil peroxidase (EPO) levels in colon lumens. Animals received dextran sodium sulphate or water for 6 days. At day 6, MPO (A; n = 12) and EPO (B; n = 12) contents in colon flushes of mice with or without colitis were determined. Levels were below detection levels in control animals. Data are presented as means (SEM). GRK, G‐protein‐coupled receptor kinase; WT, wild type. *p<0.05.
Chemotactic response of GRK6−/− granulocytes
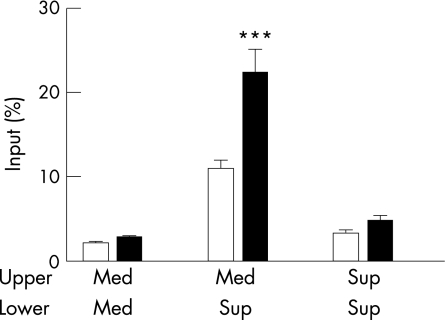
We tested the chemotactic response of GRK6−/− granulocytes to supernatants from cultures of inflamed colon. At day 3 after the start of DSS administration, colon sections from WT animals were cultured in vitro for 24 h. The culture supernatant was used as a chemoattractant for bone‐marrow‐derived granulocytes from WT and GRK6−/− animals in a transendothelial migration assay. Significantly more GRK6−/− granulocytes than WT granulocytes reached the lower well (fig 4; p<0.001). The latter indicates an increased chemotactic response of GRK6−/− cells to substances produced by the (same) inflamed colon. Chemokinesis and spontaneous migration did not differ between WT and GRK6−/− cells (fig 4).
Figure 4 Chemotactic response of granulocytes to wild‐type (WT) colon culture supernatant. WT mice received 1% dextran sodium sulphate for 3 days, and colon sections were cultured for 24 h. Colon culture supernatant (sup) or medium (med) was used as a chemoattractant in a transendothelial migration assay with WT (white bars) and GRK6−/− (black bars) granulocytes. Data are presented as means (SEM). GRK, G‐protein‐coupled receptor kinase. ***p<0.001 GRK6−/− versus WT.
Chronic colitis in GRK6−/− mice
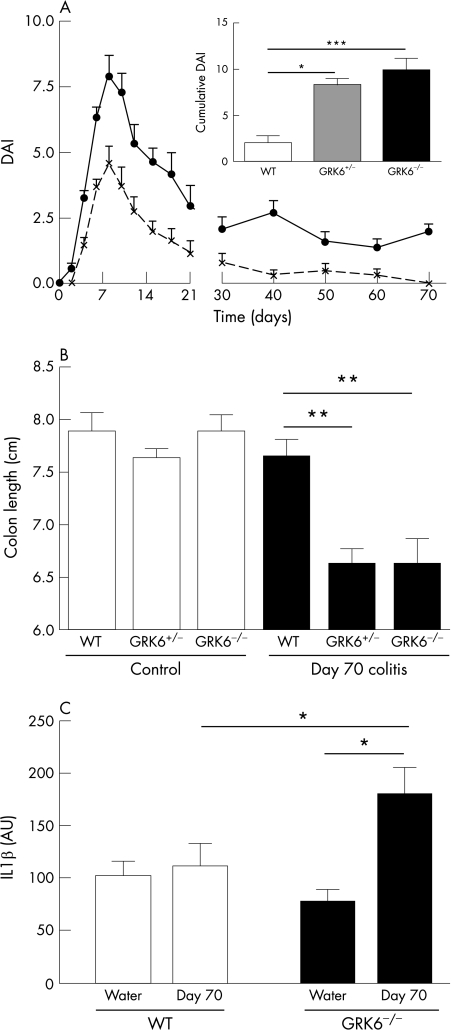
The possible effects of reduced GRK6 on disease remission were investigated by following clinical symptoms for 70 days after the induction of colitis. In WT, GRK6+/− and GRK6−/− mice, the disease activity peaked at days 8–9 and then gradually reduced (fig 5A). At day 30, only 25% of the WT animals versus 80% of the GRK6+/− and GRK6−/− animals had a disease score >2. At day 70, all the WT animals had fully recovered, but 80% of the GRK6+/− and GRK6−/− mice still had clinical symptoms of colitis. The DAI score over time and the cumulative DAI score from days 30 to 70 were significantly higher for GRK6+/− (p<0.05 vs WT) and GRK6−/− (p<0.001 vs WT) mice, indicating that normal GRK6 levels are required to prevent development of chronic colitis after a single DSS administration (fig 5A). The colon length of WT mice had returned to normal baseline length at day 70, but the colon lengths of GRK6+/− (p<0.01 vs WT) and GRK6−/− (p<0.01 vs WT) mice were still significantly shorter (fig 5B).
Figure 5 Chronic colitis in G‐protein‐coupled receptor kinase (GRK6)+/− mice. (A) Disease activity index (DAI) from wild‐type (WT; dotted line; n = 12) and GRK6−/− (continuous line; n = 10) mice over time. DAI scores over time for GRK6−/− mice significantly differed from WT animals from days 30 to 70 (repeated‐measures analysis of variance : p<0.001). Inset shows the mean cumulative DAI score calculated by summation of DAI every 10th day from day 30–70 in WT (n = 12), GRK6+/− (n = 7) and GRK6−/− (n = 10) animals. (B) Colon length on day 70. (C) Interleukin (IL)1β levels at day 70. Data are presented as means (SEM). AU, arbitrary units. * p<0.05; **p<0.01; ***p<0.001.
At day 70, colonic IL1β mRNA levels in DSS‐treated WT animals were similar to those in untreated WT animals (fig 5C). However, IL1β mRNA levels in colons of DSS‐treated GRK6−/− mice were significantly increased compared with untreated controls (fig 5C; p<0.05), indicating ongoing inflammation. Moreover, IL1β mRNA levels were significantly higher in DSS‐treated GRK6−/− mice than in DSS‐treated WT animals (p<0.05).
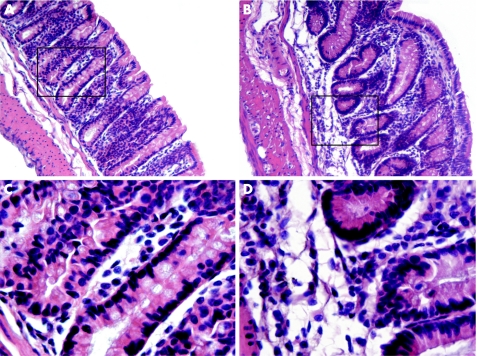
Histological examination of the colons of GRK6−/− mice at day 70 revealed cellular infiltrates in the lamina propria that were not observed in the colons of WT mice. Furthermore, mucosal oedema and crypt distortion were present in the GRK6−/− mice, whereas the crypts were well organised and the base of the tubular glands reached the muscularis mucosae in WT mice. The epithelial cell layer on the surface of the mucosa was intact in both groups (fig 6).
Figure 6 Histological sections of colons at day 70. Representative tissue sections of dextran sodium sulphate (DSS)‐treated wild‐type (WT; A, C) and G‐protein‐coupled receptor kinase 6−/− (GRK6−/−; B, D) mice at day 70. Overview at 100× (A, B) and details at 500× (C, D) magnification. Colons of the GRK6−/− mice still showed disorganisation of the crypts, whereas the crypts were well organised in WT mice. At day 70, colons of the GRK6−/− mice contained more mononuclear infiltrates in the lamina propria.
Role of acute disease severity in chronicity of colitis
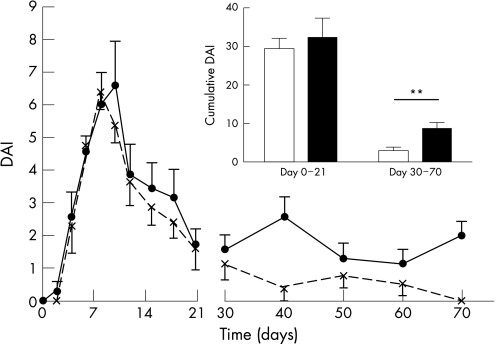
To test whether the disease severity in the acute phase determines the chronicity, we excluded from the analysis 35% of the GRK6−/− animals with the highest DAI score at the peak of disease and 35% of WT animals with the lowest DAI score at the peak of disease. This selection resulted in two groups with similar DAI scores in WT and GRK6−/− animals during the acute phase from days 0 to 21 (fig 7; p = 0.63). However, in these selected subgroups with identical disease severity during the acute phase of the disease, only GRK6−/− animals developed chronic disease, and the difference in DAI scores between GRK6−/− and WT mice from days 30 to 70 was maintained (fig 7; p<0.01). These data suggest that the development of DSS‐induced colitis into a chronic disease in GRK6−/− mice is not dependent on the increased severity during the acute phase, but on the reduction in GRK6.
Figure 7 Development of chronic colitis in G‐protein‐coupled kinase 6+/− (GRK6+/−) mice with same severity of disease as wild‐type (WT) mice. To test whether the increased severity in GRK6−/− mice caused the chronic colitis, we excluded 35% of GRK6−/− mice (continuous line; inset black bar; n = 7) with the most severe form of colitis and excluded 35% of WT mice (dotted line; inset white bar; n = 8) with very‐low‐grade colitis. This exclusion resulted in similar Disease Activity Index (DAI) scores from days 0 to 21. The DAI score of the GRK6−/− mice remained significantly different from those of the WT mice from days 30 to 70 (p<0.01). Data are presented as means (SEM). **p<0.01.
Cellular infiltrates
At day 70, MPO and EPO levels were below the detection limit in both WT and GRK6−/− mice as in the healthy controls (data not shown). Histology of the colon revealed increased mononuclear cell infiltrates in the lamina propria of the colons of GRK6−/− mice at day 70.
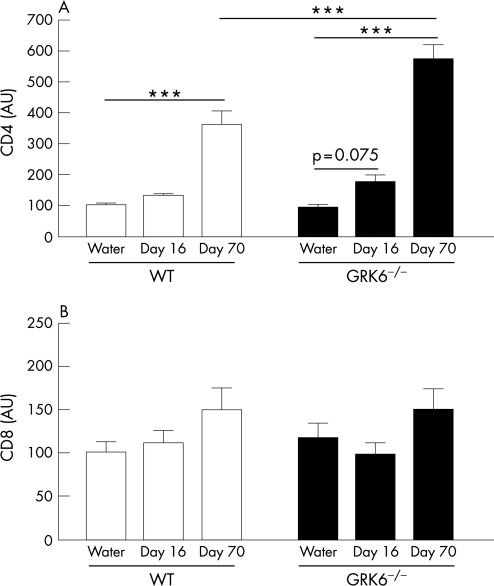
To obtain more insight into the time course and nature of these cellular infiltrates in the chronic phase of colitis in GRK6−/− animals, we determined CD4 and CD8 mRNA levels in the colon during recovery from the acute phase of colitis at day 16 and from the chronic phase at day 70. At day 16, a gradual increase in CD4 expression was observed when comparing healthy controls and DSS‐treated GRK6−/− animals (p = 0.075; fig 8A). At this time point, CD4 expression in DSS‐treated WT animals was similar to that of controls. At day 70, colons of DSS‐treated WT and GRK6−/− animals contained higher levels of CD4 than colons of controls (fig 8A; p<0.001). At day 70, CD4 expression was significantly higher in GRK6−/− mice than in WT mice (fig 8A; p<0.001). CD8 expression was similar in all groups (fig 8B).
Figure 8 CD4 and CD8 levels in the colon at days 16 and 70. Mice received 1% dextran sodium sulphate (DSS) in drinking water (n = 6–12) or water (n = 9–11) for 6 days, followed by normal drinking water. At days 16 and 70, the amount of CD4 (A) and CD8 (B) mRNA was determined by real–time reverse transcriptase PCR. Data are presented as means (SEM). AU, arbitrary units; GRK G‐protein‐coupled receptor kinase; WT, wild type. ***p<0.001.
Treg presence and function
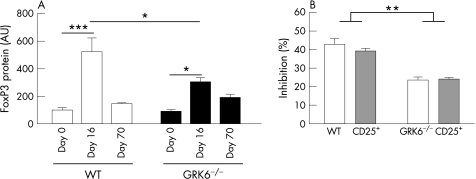
To test whether ongoing disease in GRK6−/− mice may be due to altered Treg activity, we first determined FoxP3 protein expression in the colon at day 0, 16 and 70. At day 16, FoxP3 protein expression was increased in both WT and GRK6−/− animals compared with healthy animals, but FoxP3 protein levels were significantly higher in WT animals than in GRK6−/− animals (fig 9A; p<0.05).
Figure 9 Forkhead box protein P3 (FoxP3) expression and regulatory T cell (T(reg)) function. (A) Animals received 1% dextran sodium sulphate (DSS) in drinking water (n = 7) or water (n = 10) for 6 days, followed by normal drinking water. At days 16 and 70, the amount of FoxP3 expression was determined by western blotting. (B) Percentage of inhibition of the proliferation of CD4+C25− T cells of naive wild‐type (WT; white bars) and naive G‐protein‐coupled kinase 6−/− (GRK6−/−; hatched bars) mice by CD4+CD25+ T cells of WT and GRK6−/− mice. Data are presented as mean (SEM). AU, arbitrary units. *p<0.05; **p<0.01; ***p<0.001.
Next, we determined the functional activity of Treg. GRK6−/−CD4+CD25+ Treg inhibited the proliferation of WT and of GRK6−/−CD4+CD25− T cells to a lesser extent than WT Treg did (fig 9B; p<0.01). The proliferative capacity of GRK6−/−CD4+CD25− T cells in the absence of Treg did not differ from that of WT CD4+CD25‐ T cells.
Discussion and conclusions
In this paper we show that GRK6 is involved in the regulation of the onset, severity and remission of DSS‐induced colitis. GRK6 is a kinase involved in regulating the sensitivity of GPCRs, including chemokine receptors. The acute phase of DSS‐induced colitis is associated with marked infiltration of granulocytes, and numerous studies have described that infiltrating granulocytes are a prerequisite for the manifestation of colitis. Our data show that colons from GRK6−/− mice contain more granulocytes than colons of their WT counterparts in the acute phase of DSS‐induced colitis. Many chemokines, such as KC, monocyte chemoattractant protein 1, RANTES, and LTB4, are involved in the process of attracting inflammatory cells, and these substances and their receptors contribute to determining the severity of colitis.9,10,23,24 Chemokine and chemoattractant receptors that are known to be regulated by GRK6 include chemokine (CXC motif) receptor 4, CCR5 and the LTB4 receptor.18,19,20,25,26 Moreover, we have shown previously that GRK6−/− and GRK6+/− granulocytes have an increased chemotactic response to the chemokine stromal cell‐derived factor 1 and the chemoattractant LTB4.18,19 Here we demonstrate that the chemotactic response of GRK6−/− granulocytes to factors produced by the inflamed colon is also increased. Although we do not know which chemokine produced by the colon of DSS‐treated mice is responsible for the increased chemotactic response, we propose that the increased reactivity of GRK6−/− and GRK6+/− granulocytes is responsible for the advanced onset and increased severity of DSS‐induced colitis in the acute phase of the disease.
The DSS‐induced colitis model has been described as an acute, remitting colitis model.3 Interestingly, reduced levels or absence of GRK6 prevented recovery from colitis until at least day 70 after initiation of the disease. At this time, GRK6−/− animals still had clinical symptoms of colitis, which were associated with histological changes that are characteristic of ongoing inflammation, whereas WT animals had completely recovered. In addition, we observed a decrease in colon length, increased IL1β and increased CD4 expression in GRK6−/− animals at day 70.
The question arises of how reduced GRK6 levels can facilitate the development of chronic colitis. The progression of acute colitis to chronic colitis might be mediated through continuous attraction of granulocytes to the site of inflammation. However, histological examination of the colon and the quantification of the granulocyte markers EPO and MPO revealed that only a few granulocytes were present in both WT and GRK6−/− mice at day 70.
The cellular infiltrates of GRK6−/− mice at day 70 mainly consisted of cells of lymphocytic origin. Some lymphocytic infiltrates were also still present in the colon of the clinically recovered WT animals. Quantitative analysis of the expression of CD4 and CD8 in colons at day 70 showed a higher level of CD4 in DSS‐treated GRK6−/− mice than in WT animals. These data suggest that a more pronounced infiltration of CD4 T cells may contribute to disease chronicity. In a model of chronic colitis that uses repetitive administration of DSS to induce a chronic course, it has also been reported that CD4 T cells are a major constituent of the cellular infiltrates.7 Increased sensitivity of chemokine receptors on GRK6‐deficient CD4 T cells may be responsible for the increased infiltration of CD4 cells into the colons of GRK6−/− mice.
An intriguing finding is that at day 16 the transcription factor FoxP3 is lower in colons of GRK6−/− mice than in colons of WT mice, suggesting that there are fewer Treg in colons of GRK6−/− mice. Treg have been described as a cell subset that downregulates inflammation or prevents diseases including colitis.27,28,29 Thus, the reduced expression of FoxP3 may be one of the underlying mechanisms for the development of chronic colitis in GRK6−/− mice. Moreover, our in vitro data suggest that GRK6−/−CD4+CD25+ cells have a reduced suppressive activity. Therefore, we propose that GRK6 is crucial for adequate Treg activity.
The course of DSS‐induced colitis did not differ between mice completely deficient in GRK6 and mice with a partial deletion of GRK6. We have reported previously that GRK6−/− and GRK6+/− animals show a similar increase in inflammatory activity in a model of acute inflammation of the skin.18 Similarly, we observed that mice heterozygotic for the deletion of GRK2, another kinase of the GRK family, have an advanced onset of experimental inflammatory autoimmune encephalomyelitis, an animal model for multiple sclerosis.30 Thus, even a partial reduction of the expression of kinases of the GRK family can have important consequences for the course of inflammatory diseases. These observations are especially important in the light of our findings that chronic inflammatory diseases are associated with changes in the intracellular levels of GRK2 and GRK6. The expression of these kinases in leucocytes in patients with rheumatoid arthritis or in patients with multiple sclerosis is reduced by 40–50%30,31 (and unpublished observations).
In conclusion, we have shown here that a reduction in the expression of GRK6 has marked consequences for the course of DSS‐induced colitis in mice. The novel finding that GRK6 is involved in Treg function deserves further attention. Changes in GRK6 expression in cells from patients with ulcerative colitis should be investigated to obtain more insight into the pathophysiology of inflammatory bowel disease.
Acknowledgements
We thank Dr C‐J S Edgell (University of North Carolina at Chapel Hill) for providing the Ea.hy926 cell line. This work has been funded by a bilateral cooperation program NWO/DFG grant SCH 341/11–1, 11–2.
Abbreviations
ANOVA - analysis of variance
CCR - chemokine (CC motif) receptor
DAI - Disease Activity Index
DSS - dextran sodium sulphate
EPO - eosinophil peroxidase
FoxP3 - forkhead box protein P3
GPCR - G‐protein‐coupled receptor
GRK - G‐protein‐coupled receptor kinase
IL - interleukin
KC - keratinocyte‐derived chemokine
LTB4 - leucotriene B4
MPO - myeloperoxidase
Treg - regulatory T cells
WT - wild type
Footnotes
Competing interests: None.
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998115182–205. [DOI] [PubMed] [Google Scholar]
- 2.Cooper H S, Murthy S N, Shah R S.et al Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 199369238–249. [PubMed] [Google Scholar]
- 3.Okayasu I, Hatakeyama S, Yamada M.et al A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 199098694–702. [DOI] [PubMed] [Google Scholar]
- 4.Dieleman L A, Ridwan B U, Tennyson G S.et al Dextran sulfate sodium‐induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 19941071643–1652. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya T, Fukuda S, Hamada H.et al Role of γ Δ T cells in the inflammatory response of experimental colitis mice. J Immunol 20031715507–5513. [DOI] [PubMed] [Google Scholar]
- 6.Dieleman L A, Palmen M J, Akol H.et al Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 1998114385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teramoto K, Miura S, Tsuzuki Y.et al Increased lymphocyte trafficking to colonic microvessels is dependent on MAdCAM‐1 and C‐C chemokine mLARC/CCL20 in DSS‐induced mice colitis. Clin Exp Immunol 2005139421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varona R, Cadenas V, Flores J.et al CCR6 has a non‐redundant role in the development of inflammatory bowel disease. Eur J Immunol 2003332937–2946. [DOI] [PubMed] [Google Scholar]
- 9.Andres P G, Beck P L, Mizoguchi E.et al Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate‐mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte‐associated Th2‐type immune response in the intestine. J Immunol 20001646303–6312. [DOI] [PubMed] [Google Scholar]
- 10.Tokuyama H, Ueha S, Kurachi M.et al The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non‐peptide chemokine receptor antagonist protects mice from dextran sodium sulfate‐mediated colitis. Int Immunol 2005171023–1034. [DOI] [PubMed] [Google Scholar]
- 11.Lefkowitz R J. G protein‐coupled receptors. III. New roles for receptor kinases and β‐arrestins in receptor signaling and desensitization. J Biol Chem 199827318677–18680. [DOI] [PubMed] [Google Scholar]
- 12.Pitcher J A, Freedman N J, Lefkowitz R J. G protein‐coupled receptor kinases. Annu Rev Biochem 199867653–692. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson S S. Evolving concepts in G protein‐coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 2001531–24. [PubMed] [Google Scholar]
- 14.Menard L, Ferguson S S, Zhang J.et al Synergistic regulation of beta2‐adrenergic receptor sequestration: intracellular complement of β‐adrenergic receptor kinase and beta‐arrestin determine kinetics of internalization. Mol Pharmacol 199751800–808. [PubMed] [Google Scholar]
- 15.Schlador M L, Nathanson N M. Synergistic regulation of m2 muscarinic acetylcholine receptor desensitization and sequestration by G protein‐coupled receptor kinase‐2 and β‐arrestin‐1. J Biol Chem 199727218882–18890. [DOI] [PubMed] [Google Scholar]
- 16.Chuang T T, Sallese M, Ambrosini G.et al High expression of β‐adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem 19922676886–6892. [PubMed] [Google Scholar]
- 17.Loudon R P, Perussia B, Benovic J L. Differentially regulated expression of the G‐protein‐coupled receptor kinases, βARK and GRK6, during myelomonocytic cell development in vitro. Blood 1996884547–4557. [PubMed] [Google Scholar]
- 18.Kavelaars A, Vroon A, Raatgever R P.et al Increased acute inflammation, leukotriene B4‐induced chemotaxis, and signaling in mice deficient for G protein‐coupled receptor kinase 6. J Immunol 20031716128–6134. [DOI] [PubMed] [Google Scholar]
- 19.Vroon A, Heijnen C J, Raatgever R.et al GRK6 deficiency is associated with enhanced CXCR4‐mediated neutrophil chemotaxis in vitro and impaired responsiveness to G‐CSF in vivo. J Leukoc Biol 200475698–704. [DOI] [PubMed] [Google Scholar]
- 20.Fong A M, Premont R T, Richardson R M.et al Defective lymphocyte chemotaxis in β‐arrestin2‐ and GRK6‐deficient mice. Proc Natl Acad Sci USA 2002997478–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider T, Issekutz A C. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods 19961981–14. [DOI] [PubMed] [Google Scholar]
- 22.Imada A, Ina K, Shimada M.et al Coordinate upregulation of interleukin‐8 and growth‐related gene product‐alpha is present in the colonic mucosa of inflammatory bowel. Scand J Gastroenterol 200136854–864. [DOI] [PubMed] [Google Scholar]
- 23.Ajuebor M N, Hogaboam C M, Kunkel S L.et al The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol 2001166552–558. [DOI] [PubMed] [Google Scholar]
- 24.Ajuebor M N, Zagorski J, Kunkel S L.et al Contrasting roles for CXCR2 during experimental colitis. Exp Mol Pathol 2004761–8. [DOI] [PubMed] [Google Scholar]
- 25.Gaudreau R, Le Gouill C, Venne M H.et al Threonine 308 within a putative casein kinase 2 site of the cytoplasmic tail of leukotriene B(4) receptor (BLT1) is crucial for ligand‐induced, G‐protein‐coupled receptor‐specific kinase 6‐mediated desensitization. J Biol Chem 200227731567–31576. [DOI] [PubMed] [Google Scholar]
- 26.Olbrich H, Proudfoot A E, Oppermann M. Chemokine‐induced phosphorylation of CC chemokine receptor 5 (CCR5). J Leukoc Biol 199965281–285. [DOI] [PubMed] [Google Scholar]
- 27.Mottet C, Uhlig H H, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 20031703939–3943. [DOI] [PubMed] [Google Scholar]
- 28.Denning T L, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol 20051747487–7491. [DOI] [PubMed] [Google Scholar]
- 29.Huber S, Schramm C, Lehr H A.et al Cutting edge: TGF‐β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol 20041736526–6531. [DOI] [PubMed] [Google Scholar]
- 30.Vroon A, Kavelaars A, Limmroth V.et al G protein‐coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol 20051744400–4406. [DOI] [PubMed] [Google Scholar]
- 31.Lombardi M S, Kavelaars A, Schedlowski M.et al Decreased expression and activity of G‐protein‐coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J 199913715–725. [DOI] [PubMed] [Google Scholar]