Abstract
Objective
To examine the mechanisms of action of ursodeoxycholic acid (UDCA) on gallbladder (GB) muscle cells in patients with symptomatic cholesterol gallstones (GSs) as it reduces the incidence of acute cholecystitis.
Design and patients
A double‐blind study was performed on 15 patients, 7 randomised to UDCA and 8 to placebo, treated for 4 weeks before cholecystectomy. Muscle contraction induced by cholecystokinin (CCK)‐8, acetylcholine (ACh) and potassium chloride (KCl) was determined in enzymatically isolated GB muscle cells, and cholesterol levels were determined in plasma membranes. H2O2, lipid peroxidation, platelet‐activating factor (PAF)‐like lipids, prostaglandin E2 (PGE2) and catalase activity were determined as biochemical markers of oxidative stress and inflammation in muscle cells.
Results
UDCA significantly increased GB muscle cell contraction induced by all concentrations of CCK‐8, ACh and KCl, and reduced the plasma membrane cholesterol (mean (SD) 0.32 (0.16) vs 0.72 (0.5) μmol/mg of protein) compared with placebo. In GB muscle cells, UDCA treatment significantly decreased the levels of H2O2 (4.4 (1.9) vs 13.7 (5.3) μmol/mg of protein), lipid peroxidation (malondialdehyde levels 1.3 (0.4) vs 2.52 (0.7) nmol/100 mg of protein), PAF‐like lipids (8.9 (4.9) vs 29.6 (7.1) pg/mg of protein) as well as the production of PGE2 (142 (47) vs 365 (125) pg/mg of protein) and catalase activity (14.5 (9.4) vs 35.8 (12.7) units/mg of protein) when compared with placebo.
Conclusion
These studies suggest that UDCA treatment improves GB muscle contractility by decreasing the cholesterol content in the plasma membrane of muscle cells, and the biochemical parameters of oxidative stress, thus explaining its possible therapeutic mechanisms in patients with symptoms of cholesterol GSs.
The pathogenesis of acute cholecystitis (AC) is controversial.1 It has been suggested that AC is caused by cystic duct obstruction due to gallstones (GSs).2 Cystic duct stones are found in only 14% of patients with AC, and it is not clear whether they actually obstruct the cystic duct.2 Moreover, cystic duct ligation in guinea pigs fails to cause AC, unless the bile is lithogenic with cholesterol and concentrated bile is injected into the gallbladder (GB).3,4 Ursodeoxycholic acid (UDCA) treatment in patients with symptomatic GSs decreased the incidence of biliary pain and AC compared with no treatment over an 18‐year follow‐up period.5 This therapeutic effect was independent of GS dissolution. Guinea pigs submitted to common bile duct ligation (BDL) develop AC within 2–3 days with biochemical and pathological changes similar to those found in human AC, with or without GSs.2,4 GB muscle cells have increased levels of reactive oxygen species (ROS), lipid peroxidation and prostaglandin E2 (PGE2) levels, their response to cholecystokinin (CCK)‐8, PGE2 and potassium chloride (KCl) being impaired, associated with a significant reduction in receptor binding of these ligands.4 These abnormalities were reproduced by treating normal human muscle cells with H2O2 or with hydrophobic bile acids (taurochenodeoxycholic acid, TCDC) and are prevented by pre‐treatment with PGE2 or with the free radical scavenger catalase, suggesting that hydrophobic bile acids damage receptors and calcium channels of GB muscle cells by stimulating the generation of ROS.6,7 These effects of UDCA seem to be due to the ability to neutralise the actions of hydrophobic bile acid.8 The finding that muscle cells pre‐incubated with UDCA prevent TCDC‐induced muscle cell damage and ROS production supports this possibility. These studies suggest that hydrophobic bile acids are potential aggressive factors that may initiate the inflammatory process in GBs with a permissive environment created by lithogenic bile with cholesterol.9,10 This permissive environment is characterised by GB stasis of bile due to impaired muscle contraction in response to CCK‐8 and acetylcholine (ACh) and reduced PGE2‐related cytoprotection.11,12 Both abnormalities are due to lower receptor binding of ligands caused by increased plasma membrane cholesterol that localised primarily in the caveolae where receptors seem to be sequestered.12 These abnormalities can be rapidly corrected by exposing the muscle cells in vitro to cholesterol‐free liposomes that remove the excess cholesterol from the plasma membrane.12
The present studies were, therefore, designed to examine whether the therapeutic actions of UDCA in patients with symptomatic GB stones are due to improvement in GB muscle function and reduction of biochemical markers of inflammation.
Materials and methods
Patients and experimental procedure
From March 2001 to December 2003, 15 patients (9 women, 6 men, mean (range) age 44 (28–66) years; mean (SD) body mass index 24 (5)), scheduled for elective laparoscopic cholecystectomy for symptomatic GB stones determined by ultrasound examination, agreed to enter a prospective randomised double‐blind study. All patients had recurrent episodes of biliary pain (epigastric and/or right upper quadrant steady pain, lasting >30 min). None of the patients had significant or chronic dyspeptic symptoms. Patients were randomised to receive indistinguishable capsules of UDCA (10 mg/kg per day, range 8–11.2 mg/kg per day; Sanofi‐Winthrop SA) or placebo (Sanofi‐Winthrop SA; Riells Y Viabrea, Spain) for 30 days. An independent pharmacist dispensed either active or placebo tablets according to a computer‐generated randomisation list. Patients with radio‐opaque stones, pregnant women,13 patients with diabetes, with a history of gastrointestinal disease or surgery (with the exception of appendectomy) and with clinical evidence of AC or thickened GB wall (>3 mm) or a dilated bile duct were excluded from the study. Informed consent was obtained from all patients and the ethics committee of the Campus Bio Medico University of Rome, Italy, approved the study protocol.
After 30 days of UDCA or placebo treatment, all patients underwent laparoscopic cholecystectomy; GBs were removed, and the specimens were immediately immersed in a beaker with ice‐cold oxygenated Krebs solution and taken to the laboratory. Krebs solution contains 116.6 mmol/l NaCl, 3.4 mmol/l KCl, 21.9 mmol/l NaHCO3, 1.2 mmol/l NaH2PO4, 2.5 mmol/l CaCl2, 1.2 mmol/l MgCl2 and 5.4 mmol/l glucose. After the removal of serosa and mucosa under a dissecting microscope, the muscle layer was carefully cleaned by removing the remaining connective tissue and small blood vessels and then minced to 2×2 mm squares ready for further use.
Measurement of gallbladder muscle cell contraction
Muscle cells were isolated from GBs as described elsewhere.13,14,15 Cell lengths were measured with the investigator (MPLG) blinded to the treatment received by patients. Muscle cells were then exposed to CCK‐8, ACh and KCl for 30 s. They were measured according to previous reports from our laboratory.11,12,13,14,15 Overall, 30 consecutive intact muscle cells were measured using a phase contrast microscope (model Eclipse E400; Nikon Instech, Kawasaki, Japan) and a TV camera (Nikon Instech) connected to a PowerPC computer (Hewlett‐Packard, Palo Alto, California, USA). Cell contraction was expressed as percentage shortening in initial control cell length (% shortening).
Preparation of enriched plasma membranes of GB muscle
Plasma membranes were prepared and purified by sucrose gradient centrifugation as described elsewhere.10,16
Measurements of cholesterol
Lipids were extracted from 0.3 to 0.5 mg of purified plasma membranes with a 3 ml mixture of chloroform:methanol (2:1 v/v). Cholesterol was measured by the cholesterol‐oxidase method using an Amplex Red Cholesterol Assay Kit (A‐12216; Amplex, Molecular Probes Eugene, Oregon, USA).
The assay is based on an enzyme‐coupled reaction that detects both free cholesterol and cholesteryl esters. Cholesteryl esters are hydrolysed by cholesterol esterase into cholesterol, which is then oxidised by cholesterol oxidase to yield H2O2 and the corresponding ketone product. The H2O2 is then assessed using 10‐acetyl‐3,7‐dihydroxyphenoxazine (Amplex Red reagent). Sensitivity of the kit is 80 ng/ml. A diluted control sample or cholesterol standard (50 μl) was pipetted into a separate well of a microplate. To begin the reaction, 50 μl of Amplex Red reagent/horseradish peroxidase/cholesterol oxidase/cholesterol esterase working solution was added to each well. The microplate was incubated at 37°C for 30 min (protected from light). Fluorescence was measured by a fluorescence microplate reader using a Beckman spectrophotometer DU‐7 (Beckman Instruments, Irvine, California, USA) with an excitation wave of 530 nm and emission detection at 590 nm. Cholesterol content was calculated using a standard curve, calibrated by the protein content and expressed as micromoles per milligram of protein.
Measurement of lipid peroxidation
Lipid peroxidation was measured in plasma membranes by a Colorimetric Assay kit (FR12; Oxford Biomedical Research, Oxford, Michigan, USA). Muscle squares (100 mg) were homogenised in 20 mM phosphate buffer (pH 7.4), and then 10‐µl BHT (butylated hydroxytoluene) in acetonitrile was added to 1 ml tissue homogenate to prevent the oxidation of the sample. This was centrifuged at 3000 rpm for 10 min at 4°C to remove large particles. An aliquot of the sample was used for determining the content protein. TMOP (1,1,3,3‐tetramethoxypropane in Tris‐HCL) was prepared from 0.1 to 20 µM as external standards. A 200 µl sample was added to 650 µl R1 (N‐methyl‐2‐phenylindole in acetonitrile) reagent, gently vortexed and followed by the addition of HCl 150 µl 37% (12 N) to the mixture. The mixture was mixed and incubated at 45°C for 60 min. The level of lipid peroxides was expressed as nanomoles of malondialdehyde (a secondary product of lipid peroxidation) per 100 mg protein.17
Determination of H2O2 content
GB muscle squares (100 mg) were homogenised in 5 ml phosphate‐buffered saline. It was centrifuged at 15 000 rpm for 15 min at 4°C and the supernatant was collected. H2O2 was determined using the Amplex Red hydrogen peroxide/peroxidase assay kit (A‐22188; Amplex, Molecular Probes). Amplex Red reagent is used to detect the H2O2 content fluorometrically. In the presence of peroxidase, the Amplex Red reagent reacts with H2O2 in a 1:1 stechiometry to produce the red‐fluorescent oxidation product resorufin.18 Sensitivity was 10 picomoles H2O2/100 μl. H2O2 standard reference range was from 0.1 to 100 μmol. A volume of 50 μl sample or standard was added to 50 μl working reagent in each microplate. This solution was mixed and incubated at room temperature for 30 min, then placed in a microplate reader measured at 530 nm excitation and fluorescence emission detection at 590 nm. H2O2 content was calculated from the standard curve and expressed as micromoles per milligram of protein.
Measurements of platelet‐activating‐factor‐like lipids
Platelet‐activating factors were measured using the method of Sarchielli et al.19 It was measured using [3H]–PAF scintillation proximity assay (SPA) system (TRK990; Amersham Biosciences, Princeton, New Jersey, USA). Muscle squares (100 mg) were homogenised in 10 ml methanol:chloroform (2:1(v/v)). An aliquot of homogenate was taken for the measurement of protein. PAF was extracted from the homogenate with a biphasic mixture formed by adding 5 ml each of chloroform and aqueous NaCl (1 mol/l). The mixture was shaken and then centrifuged at 5000 rpm for 10 min at 4°C to remove big particles. The chloroform extracts were collected and evaporated to dryness, and immediately re‐dissolved in 5 ml chloroform/methanol [4:1(v/v)]. The concentrated extracts were then applied to Amprep silica mini columns (Amersham Biosciences; pre‐equilibrated in chloroform) followed by a rinse with 10 ml chloroform:methanol (4:1(v/v)). PAF was then purified and eluted in 15 ml chloroform:methanol (4:1(v/v)), 9 ml of methanol and then 12 ml of H2O2 was added and shaken to form a biphasic mixture. The chloroform phase was collected and evaporated to dryness, immediately dissolved in the SPA buffer (0.05 M Tris/HCL, pH 7.4, 0.9% NaCl, 0.01% TritonX‐100, 0.1% gelatin, 2.5% Germall1) for final determination. In the SPA, the antibody bound to PAF is reacted with the SPA reagent, which contains the protein‐A bound to fluomicrospheres. Any [3H]‐PAF that is bound to the antibody will therefore be immobilised on the protein‐A fluomicrosphere which will produce light. Measurement in a β‐scintillation counter enables to calculate the amount of labelled PAF that is bound. The unlabelled PAF is then determined by interpolation from a standard curve. Sensitivity was 20 pg/tube. Data are expressed as picogram per milligram protein.
Measurement of prostaglandin E2 levels
PGE2 content was measured using an enzyme immunoassay (EIA) kit (514010; Cayman Chemical Company, Ann Arbor, Michigan, USA). Muscle squares (100 mg) were homogenised in PGE2 homogenisation buffer (0.1 M phosphate buffer (pH 7.4) containing 1 mM EDTA and 20 μg/ml indomethacin) at 4°C. An aliquot was taken for protein measurement. The homogenate was centrifuged at 15 000 rpm for 15 min at 4°C and the supernatant was used for PGE2 purification by a PGE2 affinity column (Cayman Chemical Company). The extracts were dissolved in EIA buffer (1.0 M phosphate buffer pH 7.4 containing 0.01% NaN3, 0.037% EDTA, 0.1% BSA). PGE2 concentration was quantified using a PGE2 competitive EIA kit and expressed as nanogram per milligram of protein.20
Measurement of catalase activity
The activity of catalase was measured using a catalase assay kit (707002; Cayman Chemical Company) as reported previously.11,12 It is based on the enzyme reaction with methanol in an optimal concentration of H2O2. The formaldehyde produced is measured spectrophotometrically with 4‐amino‐3‐hydrazino‐5‐mercapto‐1,2,4‐triazole (Purpald; Aldrich chemical company, Milwaukee, WI, USA) as the chromogen. Muscle squares (100 mg) were homogenised on ice with 5 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA. Formaldehyde standards were prepared from 0 to 75 µM. Bovine liver catalase was used as a positive control. To each designated well on the plate, 100 µl of the assay buffer with 30 µl methanol and 20 µl standard or either sample or positive control catalase was added. The formaldehyde concentration of the samples was obtained from the standard curve. One unit of catalase activity is defined as the amount of enzyme that caused the formation of 1.0 nmol of formaldehyde per minute per milligram of protein at 25°C.21,22
Determination of protein content
The protein content of the muscle membranes was measured using the Bio‐Rad protein assay kit (Bio‐Rad Laboratories, Melville, New York, USA). Values for each sample are means of triplicate measurements.
Statistics
Data are expressed as mean (SD). One‐ and two‐factorial repeated analysis of variance and unpaired Student's t tests were used for statistical analysis. A p value of 0.05 was considered significant.
Results
After randomisation, seven patients received UDCA and eight received placebo for 30 days (range 28–34 days). The two groups were comparable for age, sex, body mass index and GS composition. During the study, the patients did not report any pain or side effects. Analysis of the stone revealed a cholesterol content of >70% dry weight in all patients, thus, confirming that none presented pigment stones.
No significant differences in the resting cell length were observed between the groups treated with UDCA and placebo (62.37 (1.6) and 61.23 (1.5) μm, respectively).
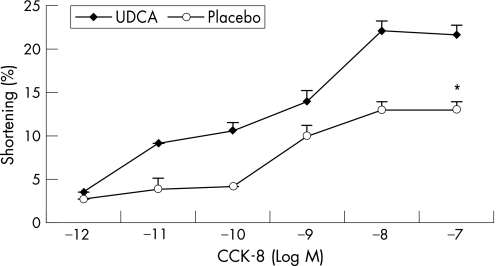
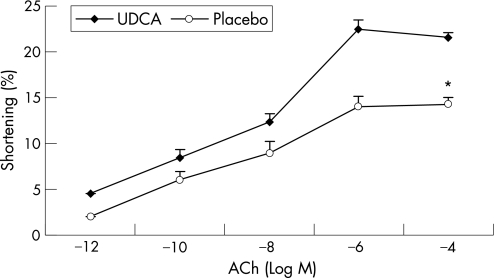
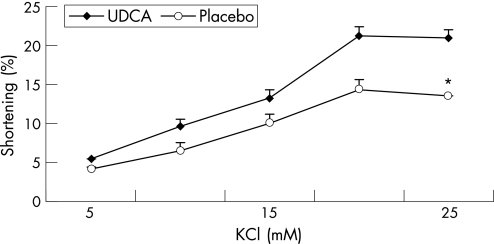
Figures 1–3 show the muscle contraction induced by increasing concentrations of CCK‐8, ACh and KCl in both groups (n = 3 in each group). UDCA treatment increased the contraction induced by all concentrations of CCK‐8 compared with placebo treatment. In the UDCA‐treated GBs, the maximal CCK‐8 concentration (10−8 M) increased the contraction from 13% (0.3%) to 22.1% (0.7%) in the placebo‐treated group (p<0.001). Treatment with UDCA also significantly improved ACh‐ and KCl‐induced muscle contraction. In the placebo‐treated patients, the maximal ACh‐induced contraction (10−6 M) was significantly lower than in UDCA‐treated patients (13.9% (0.6%) vs 22.5% (1.2%), respectively; p<0.001; fig 2). As shown in fig 3, 20 mol/l KCl caused a maximal muscle cell contraction of 14.3% (0.4%) and of 21.2% (0.9%) in the placebo‐ and UDCA‐treated group, respectively (p<0.001).
Figure 1 Dose–response studies with cholecystokinin (CCK)‐8 in dissociated muscle cells from gallbladders of patients treated with ursodeoxycholic acid (UDCA; ♦) or placebo (o). Contractions in response to all concentrations of CCK‐8 were significantly increased after treatment with UDCA compared with placebo treatment. Values are mean (SE) of three experiments. *p<0.001, analysis of variance.
Figure 2 Dose–response studies with acetylcholine (ACh) in muscle cells from gallbladders of patients treated with ursodeoxycholic acid (UDCA; ♦) or placebo (o). Contractions in response to all doses of ACh were significantly increased after treatment with UDCA compared with those after placebo treatment. Values are mean (SE) of three experiments. *p<0.001, analysis of variance.
Figure 3 Dose–response studies with KCl in muscle cells from gallbladders of patients treated with ursodeoxycholic acid (UDCA; ♦) or placebo (o). Contractions in response to all doses of KCl were increased after UDCA compared with placebo treatment. Values are mean (SE) of three experiments. *p<0.001, analysis of variance.
The cholesterol content in the plasma membranes of muscle cells from UDCA‐treated patients was significantly lower than that from the placebo group (table 1, p<0.001).
Table 1 Cholesterol levels and biochemical markers of oxidative stress and inflammation in muscle cells from gallbladders of patients treated with ursodeoxycholic acid or placebo.
| Gallbladder muscle cells* | Placebo group (n = 8 patients) | UDCA group (n = 7 patients) |
|---|---|---|
| Cholesterol content in plasma membranes (µmol/mg protein) | 0.72 (0.5) | 0.32 (0.16) |
| H2O2 levels (μmol/mg protein) | 13.7 (5.3) | 4.4 (1.9) |
| MDA levels (nmol/100 mg protein) | 2.52 (0.7) | 1.3 (0.4) |
| PAF‐like lipids levels in plasma membranes (pg/mg protein) | 29.6 (7.1) | 8.9 (4.9) |
| PGE2 production (pg/mg protein) | 365 (125) | 142 (47) |
| Catalase activity (units/mg protein) | 35.8 (12.7) | 14.5 (9.4) |
MDA, malondialdehyde; PAF, platelet‐activating factor; PGE2, prostaglandin E2; UDCA, ursodeoxycholic acid.
*Unpaired student's t test gave p<0.001 for all.
Values are expressed as mean (SD).
Measurements of biochemical parameters of oxidative stress and inflammation were also obtained in muscle cells from patients treated with UDCA or placebo. UDCA treatment significantly decreased the levels of H2O2 (table 1); it also lowered the levels of lipid peroxidation compared with placebo treatment (table 1), as assessed by MDA levels (p<0.001). Levels of PAF‐like lipids (table 1) were reduced by UDCA treatment (p<0.001). The production of PGE2 significantly decreased after UDCA treatment compared with the placebo treatment (p<0.001; table 1). Catalase activity was also reduced by UDCA compared with placebo (table 1, p<0.001).
Discussion
Results of this double‐blind, randomised, 4‐week study comparing the effects of UDCA with those of placebo in patients scheduled to undergo cholecystectomy for symptomatic GB stones revealed that pre‐treatment with UDCA restores normal contraction of GB muscle cells, induced by CCK‐8 and ACh, that activate receptor‐G protein‐coupled receptors, and, by KCl, a calcium channel‐dependent agonist. These results are consistent with the findings from a non‐randomised study showing improved GB muscle strip contraction in patients treated with UDCA for 3 weeks compared with patients not receiving the treatment.23 Presently, the effect of UDCA on in vivo GB motor function is controversial, reporting either no effect or impairment in the emptying of GB, as determined by ultrasonographic and scintigraphic studies.24,25,26,27,28 These studies have been carried out without taking into consideration the recent findings that the GB volume has bellow‐like fluctuations characterised postprandially, by a continuous and rapidly alternating phases of emptying and filling, instead of the traditionally assumed pattern of two steady and consecutive phases of emptying and filling of GB.29,30,31 When the continuous GB wall fluctuations are considered to evaluate the GB bile washout, a recent randomised study has shown, using Computer Fluido‐dynamics, that short‐time UDCA treatment improves GB bile turnover in patients with GS.32 However, so far, no studies have focused on the mechanism by which UDCA improves GB contractility. Data emerging from the present studies show that the normal muscle contraction observed after treatment with UDCA was associated with a reduction in the cholesterol levels in the plasma membrane and in the biochemical markers of oxidative stress and inflammation.
Previous studies carried out by other researchers and also by our group have shown that human GBs with cholesterol stones contract poorly in response to agonists that stimulate G‐protein‐coupled receptors such as CCK‐8, ACh, PGE2 and to calcium channel‐dependent KCl.9,10 This impaired muscle contraction is associated with the increased plasma membrane cholesterol localised in the caveolae,12 high‐cholesterol domains containing caveolin proteins and a variety of molecules involved in signal transduction pathways.14,15 The impaired contraction of muscle cells from GBs with lithogenic bile and excess cholesterol is due to lower ligand binding caused by the sequestration of receptors in the caveolae.12 As a result of reduced binding, these muscle cells also have an impaired PGE2‐dependent cytoprotection with decreased levels of catalase activity.11,12 PGE2 stimulates the activity of catalase in both human and guinea pig GB muscle cells and is significantly reduced in muscle cells, with high cholesterol levels in the plasma membrane. All these abnormalities are corrected when muscle cells are incubated with cholesterol‐free liposomes that remove the excess cholesterol from the plasma membrane. Thus, these findings suggest that high levels of cholesterol in the plasma membrane create a permissive environment characterised by defective muscle contraction and GB stasis as well as impaired PGE2‐dependent cytoprotection.
The therapeutic effect of UDCA in patients with symptom of GB stones continues to be controversial: an important 18‐year study showed a significant decrease in the incidence of AC compared with no treatment.5 By contrast, a 3‐month randomised placebo‐controlled study showed that UDCA did not exert any beneficial effect on biliary pain or complications.33 However, there are significant differences in the recurrence rates of biliary pain and the need for cholecystectomy between these two studies. Tomida et al5 reported recurrence rates of <10% in UDCA compared with 40% in placebo at 4 years. By contrast, in the most recent clinical trial, the need for cholecystectomy after 100 days of UDCA or placebo treatment was almost 75%.33 These differences suggest that UDCA may not be effective in patients with more advanced chronic inflammatory GB disease.
Our findings show that UDCA treatment restores GB muscle functions and reduces the biochemical markers of oxidative stress and inflammation may support, and partially explain, the beneficial effects in patients with symptoms of GB stones, which were independent of gallstone dissolution.5 UDCA may achieve these effects by reducing the levels of plasma membrane cholesterol, thus restoring the normal GB environment.8,34 The mechanism by which UDCA decreases the levels of plasma membrane cholesterol remains to be elucidated. It is well known, however, that bile cholesterol diffuses through the GB mucosa,9 is incorporated by the plasma membrane of muscle cells and can be easily removed with cholesterol‐free liposomes in vitro. UDCA treatment corrects bile abnormalities in patients with cholesterol GSs and microlithiasis, as demonstrated by the reduction in total and vesicular cholesterol, formation of cholesterol crystals and bile viscosity.35,36,37 It is thus conceivable that the lower total bile cholesterol and the increased incorporation of cholesterol into micelles by increased concentration of non‐aggressive hydrophilic bile acids may reduce cholesterol diffusion from the GB bile and, eventually, allow muscle cells to dispose of the excessive plasma membrane cholesterol. However, further studies are needed to demonstrate this hypothesis.
UDCA may decrease the biomarkers of oxidative stress and inflammation by neutralising the actions of hydrophobic bile acids that constitute the largest percentage of bile acids in human bile. This hypothesis is supported by the UDCA pre‐treatment on experimental AC in guinea pigs induced by common BDL. Pre‐treatment with TCDC prior to the common BDL worsened the muscle injury, whereas pre‐treatment with UDCA prevented the development of AC.8 These prophylactic effects of UDCA seem to be due to the ability to neutralise the actions of hydrophobic bile acid. Hydrophobic bile acids damage GB muscle by stimulating the generation of H2O2.7 ROS cause lipid peroxidation and formation of PAF‐like lipids stimulating PGE2 synthesis, which, in turn, activates catalase, thus preventing further damage to membrane proteins including receptors.38 Pre‐incubating muscle cells with UDCA prevents the damage induced by subsequent exposure to hydrophobic bile acids. High plasma membrane cholesterol impairs PGE2‐dependent cytoprotection of muscle cells against the action of ROS because of the impaired PGE2 binding to its receptors resulting in decreased catalase activation induced by H2O2 or hydrophobic bile acids.11,12
In summary, the present study shows that UDCA improves GB muscle contractility by decreasing cholesterol content in the plasma membranes and the biochemical parameters of oxidative stress, thus, explaining its therapeutic effects in patients with symptoms of cholesterol GSs. The obtained data support the hypothesis that lithogenic bile with excess cholesterol creates a permissive environment in the GBs altering the normal balance between hydrophobic bile acids and GB protective mechanisms. Bile acids stimulate the formation of ROS, capable of initiating inflammatory processes and cholecystitis. Thus, UDCA, by reducing the excess cholesterol and by neutralising the hydrophobic bile acids, restores the balance between aggressive biliary factors and GB protective mechanisms. These preliminary results, therefore, should encourage further larger studies to support clinical trials showing that UDCA prevents AC, and suggest that this hydrophilic acid could be an alternative therapeutic approach in high‐surgical‐risk patients with symptoms of GB stones.
Acknowledgements
We thank Sanofi‐Winthrop SA for providing the drug and placebo used in the study.
Abbreviations
AC - acute cholecystitis
ACh - acetylcholine
BDL - bile duct ligation
CCK - cholecystokinin
EIA - enzyme immunoassay
GB - gallbladder
GS - gallstone
KCl - potassium chloride
PAF - platelet‐activating factor
PGE2 - prostaglandin E2
ROS - reactive oxygen species
SPA - scintillation proximity assay
TCDC - taurochenodeoxycholic acid
UDCA - ursodeoxycholic acid
Footnotes
Funding: This study was partially supported by NIH grant RO1‐DK27389‐19 and was carried out in accordance with the Declaration of Helsinki.
Competing interests: None.
References
- 1.Roslyn J J, DenBesten L, Thompson J E J.et al Roles of lithogenic bile and cystic duct occlusion in the pathogenesis of acute cholecystitis. Am J Surg 1980140126–130. [DOI] [PubMed] [Google Scholar]
- 2.Strasberg S M. Acute calculous cholecystitis. In: Haubrich WS, Schaffner F, Berk JE, eds. Gastroenterology. Philadelphia: Saunders, 1995, 2635–64; 2665–73
- 3.Parkman H P, Bogar L J, Bartula L L.et al Effect of experimental acalculous cholecystitis on gallbladder smooth muscle contractility. Dig Dis Sci 1999442235–2243. [DOI] [PubMed] [Google Scholar]
- 4.Xiao Z L, Chen Q, Biancani P.et al Abnormalities of the gallbladder muscle associated with acute inflammation in guinea pigs. Am J Physiol 2001281490–497. [DOI] [PubMed] [Google Scholar]
- 5.Tomida S, Abei M, Yamaguchi T.et al Long‐term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: a cohort analysis. Hepatology 1999306–13. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Z L, Rho A K, Biancani P.et al Effects of bile acids on the muscle functions of guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol 200228387–94. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Z L, Andrada M J, Biancani P.et al Reactive oxygen species (H2O2): effects on the gallbladder muscle of guinea pigs. Am J Physiol Gastrointest Liver Physiol 2002282300–306. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Z L, Biancani P, Carey M C.et al Hydrophilic but not hydrophobic bile acids prevent gallbladder muscle dysfunction in acute cholecystitis. Hepatology 2003371442–1450. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Amaral J, Biancani P.et al Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology 1999116678–685. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Z L, Chen Q, Amaral J.et al CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol 19992761401–1407. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Z L, Biancani P, Behar J. Role of PGE2 on gallbladder muscle cytoprotection of guinea pigs. Am J Physiol Gastrointest Liver Physiol 200428682–88. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Z L, Amaral J, Biancani P.et al Impaired cytoprotective function of muscle in human gallbladders with cholesterol stones. Am J Physiol Gastrointest Liver Physiol 2005288525–532. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Amaral J, Oh S.et al Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology 1997113930–937. [DOI] [PubMed] [Google Scholar]
- 14.Behar J, Rhim B, Thompson W R.et al Inositol trisphosphate restores impaired human gallbladder motility associated with cholesterol stones. Gastroenterology 1993104563–568. [DOI] [PubMed] [Google Scholar]
- 15.Yu P, Chen Q, Harnett K M.et al Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am J Physiol 1995269659–665. [DOI] [PubMed] [Google Scholar]
- 16.Shaw M J, Hadac E M, Miller L J. Preparation of enriched plasma membranes from bovine gallbladder muscularis for characterization of cholecystokinin receptors. J Biol Chem 19872914313–14318. [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 197995351–358. [DOI] [PubMed] [Google Scholar]
- 18.Zhou M, Diwu Z, Panchuk‐Voloshina N.et al A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 1997253162–168. [DOI] [PubMed] [Google Scholar]
- 19.Sarchielli P, Alberti A, Coppola F.et al Platelet‐activating factor (PAF) in internal jugular venous blood of migraine without aura patients assessed during migraine attacks. Cephalalgia 200424623–630. [DOI] [PubMed] [Google Scholar]
- 20.Kelly R W, Graham B J M, O'Sullivan M J. Measurement of PGE2 as the methyl oxime by radioimmunoassay using a novel iodinated label. Prostaglandins Leukot Essent Fatty Acids 198937187–191. [DOI] [PubMed] [Google Scholar]
- 21.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem 19703430–38. [DOI] [PubMed] [Google Scholar]
- 22.Nieto N, Fernandez M I, Torres M I.et al Dietarymonounsaturated n‐3 and n‐6 long‐chain polyunsaturated fatty acids affect cellular antioxidant defense system in rats with experimental ulcerative colitis induced by trinitrobenzene sulfonic acid. Dig Dis Sci 1998432676–2687. [DOI] [PubMed] [Google Scholar]
- 23.van de Heijning B J, van de Meeberg P C, Portincasa P.et al Effects of ursodeoxycholic acid therapy on in vitro gallbladder contractility in patients with cholesterol gallstones. Dig Dis Sci 199944190–196. [DOI] [PubMed] [Google Scholar]
- 24.Rothstein R D, Brugge W R, Malet P F. Effect of extracorporeal shock wave lithotripsy and ursodeoxycholic acid on gallbladder motility. Dig Dis Sci 1993381712–1717. [DOI] [PubMed] [Google Scholar]
- 25.Portincasa P, DiCiaula A, Palmieri V.et al Tauroursodeoxycholic acid, ursodeoxycholic acid and gallbladder motility in gallstone patients and healthy subjects. Ital J Gastroenterol 199628111–113. [PubMed] [Google Scholar]
- 26.Festi D, Frabboni R, Bazzoli F.et al Gallbladder motility in cholesterol gallstone disease. Effect of ursodeoxycholic acid administration and gallstone dissolution. Gastroenterology 1990991779–1785. [DOI] [PubMed] [Google Scholar]
- 27.Forgacs I C, Maisey M N, Murphy G M.et al Influence of gallstones and ursodeoxycholic acid therapy on gallbladder emptying. Gastroenterology 198487299–307. [PubMed] [Google Scholar]
- 28.Colecchia A, Mazzella G, Sandri L.et al Ursodeoxycholic acid improves gastrointestinal motility defects in gallstone patients. World J Gastroenterol 2006125336–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jazrawi R P, Pazzi P, Petroni M L.et al Postprandial gallbladder motor function: refilling and turnover of bile in health and in cholelithiasis. Gastroenterology 1995109582–591. [DOI] [PubMed] [Google Scholar]
- 30.Pallotta N, Corazziari E, Scopinaro F.et al Noninvasive estimate of bile flux through the gallbladder in humans. Am J Gastroenterol 1998931877–1885. [DOI] [PubMed] [Google Scholar]
- 31.Cicala M, Guarino M P, Vavassori P.et al Ultrasonographic assessment of gallbladder bile exchanges in healthy subjects and in gallstone patients. Ultrasound Med Biol 2001271445–1450. [DOI] [PubMed] [Google Scholar]
- 32.Guarino M P, Carotti S, Sarzano M.et al Short‐term ursodeoxycholic acid treatment improves gallbladder bile turnover in gallstone patients: a randomised trial. Neurogastroenterol Motil 200517680–686. [DOI] [PubMed] [Google Scholar]
- 33.Venneman N G, Besselink M G, Keulemans Y C.et al Ursodeoxycholic acid exerts no beneficial effect in patients with symptomatic gallstones awaiting cholecystectomy. Hepatology 2006431276–1283. [DOI] [PubMed] [Google Scholar]
- 34.Yu P, Chen Q, Biancani P.et al Membrane cholesterol alters gallbladder muscle contractility in prairie dogs. Am J Physiol 199627156–61. [DOI] [PubMed] [Google Scholar]
- 35.Fischer S, Muller I, Zundt B Z.et al Ursodeoxycholic acid decreases viscosity and sedimentable fractions of gallbladder bile in patients with cholesterol gallstones. Eur J Gastroenterol Hepatol 200416305–311. [DOI] [PubMed] [Google Scholar]
- 36.Kano M, Shoda J, Irimura T.et al Effects of long‐term ursodeoxycholate administration on expression levels of secretory low‐molecular‐weight phospholipases A2 and mucin genes in gallbladders and biliary composition in patients with multiple cholesterol stones. Hepatology 199828302–313. [DOI] [PubMed] [Google Scholar]
- 37.Sharma B C, Agarwal D K, Dhiman R K.et al Bile lithogenicity and gallbladder emptying in patients with microlithiasis: effect of bile acid therapy. Gastroenterology 1998115124–128. [DOI] [PubMed] [Google Scholar]
- 38.Guarino M P, Xiao Z L, Biancani P.et al PAF‐like lipids‐ and PAF‐induced gallbladder muscle contraction is mediated by different pathways in guinea pigs. Am J Physiol Gastrointest Liver Physiol 20032851189–1197. [DOI] [PubMed] [Google Scholar]