Abstract
Objective
To determine ephrinB1, ephrinB2 and EphB1 expression in the optic nerve head (ONH) and retina of monkeys with glaucoma and in human ONH astrocytes.
Methods
Using immunohistochemistry, the localisation of ephrinB1, ephrinB2 and EphB1 was determined in the ONH and retina bilaterally in monkeys with monocular laser‐induced glaucoma. RT‐PCR, western blot and immunocytochemistry were used to study ephrinB1, ephrinB2 and EphB1 expression in cultured human ONH astrocytes from donors with and without glaucoma.
Results
There was an increase in ephrinB1 and EphB1 expression in mild to moderate glaucoma. In the ONH, both ephrinB1 and EphB1 were localised to astrocytes and EphB1 was also localised to lamina cribrosa cells and perivascular cells. In the retina, ephrinB1 localised to Muller cells and astrocytes, and EphB1 was found in retinal ganglion cells. In ONH astrocytes in humans with glaucoma, ephrinB1 and EphB1 were up‐regulated but barely present in donors without glaucoma.
Conclusions
Ephrins are activated in early and moderate glaucoma in the ONH and retina. We postulate that the up‐regulation of Eph/ephrin pathway may play a protective role by limiting axonal damage and inflammatory cell invasion. Loss of ephrin signalling in advanced glaucoma may explain macrophage activation.
Glaucoma is the second leading cause of blindness globally1,2 and millions of people worldwide are bilaterally blind from glaucoma.3,4 Although ocular hypertension is a recognised risk for glaucomatous damage,5,6 the existence of normal tension glaucoma and patients with ocular hypertension without glaucoma indicates that other factors might be involved in the pathogenesis of glaucomatous optic neuropathy, either damaging the retinal ganglion cell axons directly or rendering the optic nerve head (ONH) more sensitive to intraocular pressure (IOP).7
Reactive astrocytes in the ONH play a role in glaucomatous optic neuropathy.8,9,10 Glial activation in glaucomatous eyes may initially represent a cellular attempt to limit the extent of neuronal injury and to promote tissue repair,11 but reactive astrocytes may also have noxious effects on optic nerve axons by creating mechanical injury and/or changing the neuronal microenvironment.12,13 As part of the changes from quiescent astrocytes to reactive astrocytes, glaucomatous ONH astrocytes exhibit differential expression of a large number of genes including upregulation of ephrinB1 and ephrinB2 in human glaucomatous astrocytes compared to normal age matched controls.14
Eph kinases constitute the largest family of receptor protein tyrosine kinases: they are divided into EphA (EphA1 to EphA8) and EphB (EphB1 to EphB6) according to their sequence homologies and ligand‐binding specificities.15 Eph ligands, termed ephrins, are expressed mainly on the cell surface. The interaction between EphB receptor and ephrinB ligand results in bidirectional signalling, which generates repulsive forces between cells.16 The Eph signalling pathway has been well studied in the central nervous system, where many of the Eph kinases are abundantly expressed.
Ephrins participate in neural development17,18 and in axonal mapping during retinal embryogenesis,19 in vascular development, tissue‐border formation, cell migration and synaptic plasticity, and regulate changes in cell shape.20 Bundesen et al21 demonstrated that cell contact‐mediated bidirectional signalling between ephrinB2 on reactive astrocytes and EphB2 on meningeal fibroblasts participates in formation of the glial scar and the exclusion of meningeal fibroblasts from the injured spinal cord. There is also EphB3 overexpression in astrocytes and motor neurons after spinal cord injury.22
In the present study, we examined the distribution of the receptor EphB1 and its ligands, ephrinB1 and ephrinB2, in astrocytes of the glaucomatous ONH. In glaucomatous neuropathy there is no typical glial scar formation, thus a role for the ephrin pathway may be to provide boundaries to astrocyte migration into and to preserve the blood–nerve barrier.
Materials and methods
Subjects and tissue preparation
Monkey tissue
Ocular tissue from seven rhesus monkeys (Macaca mulatta), ages 3–23 years, with monocular experimental glaucoma, was used for ephrinB1, ephrinB2 and EphB1 detection in the ONH and retina (table 1). Studies were performed following guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Subjects, surgical procedure and tissue processing were described previously in detail.23 Briefly, unilateral, chronic elevated IOP was induced in monkeys by laser scarification of the trabecular meshwork. IOP was monitored weekly with an applanation tonometer and maintained within 24–46 mmHg for 12–32 weeks. ONH damage was assessed by stereoscopic slit‐lamp biomicroscopy and fundus photography and by confocal scanning laser ophthalmoscopy. In each animal, the contralateral eye served as normal control.
Table 1 Monkey data.
| Monkey n/sex/age (y) | Diagnosis | Duration* (weeks) | IOP (mmHg) mean + SEM | C/D ratio | Axonal loss (%) |
|---|---|---|---|---|---|
| 1/M/3 | Mild G | 22 | E: 27.04±1.9 | E: 0.3 | 25 |
| C: 15.39±0.4 | C: 0.1 | ||||
| 2/M/3 | Mild G | 27 | E: 18.8±4.0 | E: 0.2 | 28 |
| C: 13.6±2.1 | C: 0.1 | ||||
| 3/F/5 | Mild G | 32 | E: 24.2±1.2 | E: 0.2 | 15 |
| C: 16.6±1.3 | C: 0.2 | ||||
| 4/F/20 | Moderate G | 18 | E: 37.7±2.3 | E: NV | 48 |
| C: 17.7±0.5 | C: 0.2 | ||||
| 5/F/5 | Moderate G | 36 | E: 29.4±13.0 | E: 0.7 | 58 |
| C: 18.9±2.9 | C: 0.2 | ||||
| 6/F/23 | Advanced G | 12 | E: 46.1±5.8 | E: 1.0 | 82 |
| C: 12.8±0.9 | C: 0.2 | ||||
| 7/F/20 | Advanced G | 18 | E: 40.6±2.5 | E: 1.0 | 70 |
| C: 20.6±0.6 | C: 0.0 |
*Length of time between initial intraocular pressure elevation and sacrifice.
C, control; E, experiment; G, experimental glaucoma; NV, not visible.
The monkeys were perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate‐buffered saline (PBS) pH 7.4, following deep pentobarbital anaesthesia. After enucleation the eyes were immersed in 4% paraformaldehyde for 12–24 hours and the ONH (with 2–3 mm of myelinated nerve) were dissected from surrounding tissues. Tissue was washed in 0.2% glycine in PBS and processed for paraffin embedding. The retina and optic nerve were taken for immunohistochemistry. Control samples were taken at same distances from the globe. Optic nerve damage was evaluated on cross‐section myelinated optic nerves images and stained with p‐phenylenediamine as described.24,25,26
Human optic nerve head astrocytes
Eight eyes (ages 46–76 years) without glaucoma with no history of eye disease, diabetes, or neurodegenerative disease were obtained from eye banks throughout the USA through National Disease Research Interchange (NDRI) and the Mid‐America Transplant Services. Eyes from five donors (ages 56–83 years) with well‐documented primary open angle glaucoma (POAG) were obtained through NDRI. The eyes with glaucoma had ophthalmic histories evaluated by an ophthalmologist to ascertain POAG and degree of damage. The cause of death for all donors was myocardial infarction or cardiopulmonary failure. Cultures of astrocytes were established as described.27 Astrocytes were grown in Dulbecco's modified Eagle's medium: Nutrient Mixture F‐12 (1:1) with 5% fetal bovine serum (FBS) in 100 mm dishes for RNA and protein isolation; for immunocytochemistry, astrocytes were grown on coverslips in 35 mm dishes with six wells.
Immunostaining
EphrinB1, ephrinB2 and EphB1 immunoreactivity was detected on 6 μm sagittal sections of retina, ONH and myelinated optic nerves. We used rabbit polyclonal antibodies against ephrinB1 and ephrinB2 (1:100) and EphB1 (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). We performed double immunohistochemistry using glial acidic fibrillar protein (GFAP) (1:200; Sigma, St Louis, MO, USA) as an astrocyte and Muller cells marker in eyes with glaucoma. We used secondary antibodies, Alexa 488 and Alexa 568, labelled (1:400; Molecular Probes, Eugene, OR, USA). Sections of normal and experimental eyes were stained simultaneously to control for variations in immunostaining. Cells grown on coverslips were fixed in 4% paraformaldehyde in PBS and were processed for indirect immunofluorescence staining as previously described in detail.28 Cells were incubated with the same primary antibodies described earlier. For negative controls, non‐immune serum replaced the primary antibodies. Samples were mounted on slides using Vectashield medium (Vector Laboratories, Burlingame, CA, USA). We stained two coverslips per astrocyte culture derived from five donors with glaucoma and five donors with POAG. Slides were examined in a Nikon Optiphot‐2 microscope equipped with epifluorescent illumination (Tokyo, Japan), and images were recorded using a digital camera (Spot Diagnostic Instruments, Sterling Heights, MI, USA) and stored as computer files using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA, USA).
Real‐time quantitative RT‐PCR (qRT‐PCR)
Cytoplasmic RNA was isolated from ONH astrocytes as previously described.14 custom primers specific to ephrinB1, ephrinB2 and EphB1 (table 2) were designed using the Primer Express program (PE Applied Biosystems, Forster City, CA, USA). Each amplicon crossed at least one intron to prevent genomic DNA amplification. Primer quality (lack of primer–dimer amplification) was confirmed by melting curve analysis. A total of 5 μl of 1:5 to 1:20 diluted cDNA was used for reaction with 2x Bio‐Rad SYBR Green Supermix in 50 μl, and quantitative RT‐PCR was performed by monitoring in real time the increase of SYBR Green fluorescence using the iCycler (Bio‐Rad Laboratories, Hercules, CA, USA). Relative quantitation of gene expression was performed using the standard curve method. Values were then normalised to relative amounts of 18S RNA. Experiments were carried out in triplicate in ONH astrocyte cultures from five donors with POAG and eight donors without glaucoma. The results were expressed as the means ± SD of the relative quantity of normalised mRNA and significant differences between the means were set at p<0.05 (Student's t test).
Table 2 EphrinB1, ephrinB2 and EphB1 primers.
Western blots
Western blot analysis was carried out as previously described.29 Briefly, cell lysates were prepared from ONH astrocytes grown on 100 mm plates to ∼95% confluence using lysate buffer (50 mM Tris‐HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.05% sodium azide, Roche protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN, USA)). For nuclear proteins, cell pellets were washed with lysis buffer (20 mM HEPES, pH 7.0, 10 mM KCl, 2 mM MgCl2, 0.3% IGEPAL CA‐630), and nuclear proteins were extracted at 4°C overnight in 50 μl lysis buffer with 0.5 M NaCl. Protein concentration was determined by the Bio‐Rad Protein Assay Kit. A total of 10 μg proteins per lane were run on 4–15% Tris‐HCl SDS‐PAGE and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies: polyclonal anti‐ephrinB1, ephrinB2 and EphB1 (1:1000), followed by secondary antibody conjugated to horseradish peroxidase for 1.5 h. For detection we used enhanced chemiluminescence system (Amersham Pharmacia, USA). Mouse anti‐αActin (1:5000; Sigma) and rabbit anti‐human histone H2B (1:200; Santa Cruz, USA) served as loading controls for cytoplasmic and nuclear protein, respectively (not shown).
Results
Monkey tissue
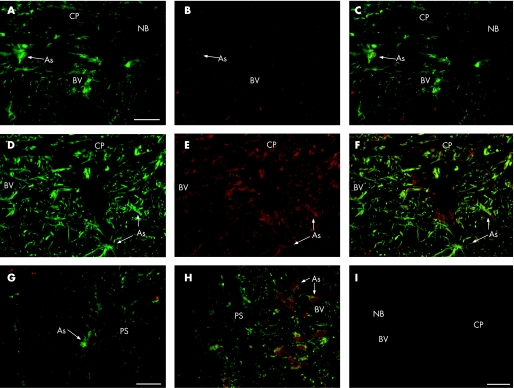
Double immunostaining for ephrinB1 and GFAP demonstrated that the expression of ephrinB1 in the adult monkey ONH without glaucoma was low, localised to ONH astrocytes (fig 1A–C). In the monkey with laser‐induced glaucoma, the expression of ephrinB1 was markedly increased in the ONH and retina, specifically in the nuclei and cytoplasm of ONH astrocytes (fig 1D–F), with a strong perivascular enhancement. The ephrinB1 expression was also present in astrocytes in the glaucomatous optic nerve just adjacent to the lamina cribrosa (fig 1G–H) surrounding the vasculature.
Figure 1 EphrinB1 expression in optic nerve head (ONH) with and without glaucoma (monkey number 3). Double immunofluorescence staining with glial acidic fibrillar protein (GFAP; green) and ephrinB1 (red) of sagittal sections shows a strong enhancement of the ephrinB1 expression in the astrocytes (As) of ONH with glaucoma (D, E, F) compared to (A, B, C) ONH without glaucoma. Note the strong expression of ephrinB1 in perivascular astrocytes. Staining of monkey retrolaminar optic nerve shows a strong enhancement of the ephrinB1 expression in astrocytes of ONH with glaucoma (H) compared to ONH without glaucoma (G), particularly in perivascular astrocytes. Note that ephrinB1 appears in the nuclei and cytoplasm of glaucomatous astrocytes. (I) Negative control immunostaining of GFAP and ephrinB1 showing no staining in the monkey ONH with glaucoma. Scale Bar, 40 μm. BV, blood vessel; CP, cribriform plates; NB, nerve bundle; PS, pial septa.
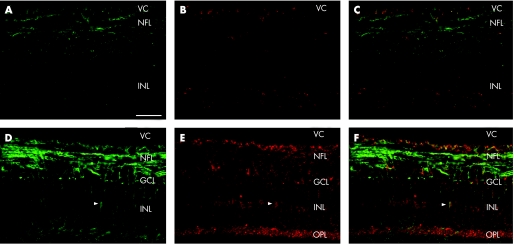
EphrinB1 expression in the normal retina was low and localised to astrocytes in the nerve fibre layer (fig 2A–C). In the monkey glaucomatous retina, ephrinB1 was found in astrocytes and Muller cells of the outer plexiform layer, inner nuclear layer, retinal ganglion cell layer and nerve fibre layer (fig 2D–F). EphrinB1 immunoreactivity was stronger in eyes with mild to moderate glaucomatous damage. In eyes with severe glaucomatous damage, ephrinB1 expression was faintly positive in astrocytes of the nerve fibre layer, resembling ephrinB1 expression in normal retina (not shown).
Figure 2 EphrinB1 expression in retinas with and without glaucoma (monkey number 5). Double immunofluorescence staining with glial acidic fibrillar protein (GFAP; green) and ephrinB1 (red) of sagittal sections showed low expression of ephrinB1 in the monkey retina without glaucoma (A, B, C) and localised to astrocytes in the nerve fibre layer. In the glaucomatous monkey retina (D, E, F), ephrinB1 is expressed in Muller cells (arrowheads) and astrocytes of the nerve fibre layer, retinal ganglion cell layer, inner nuclear layer and outer plexiform layer. Scale bar: 40 μm.
EphrinB2 expression in monkey ONH and retina with and without glaucoma was very low, limited to ONH astrocytes and the nerve fibre layer of the retina (not shown), and there was no differential staining between tissue with and without glaucoma.
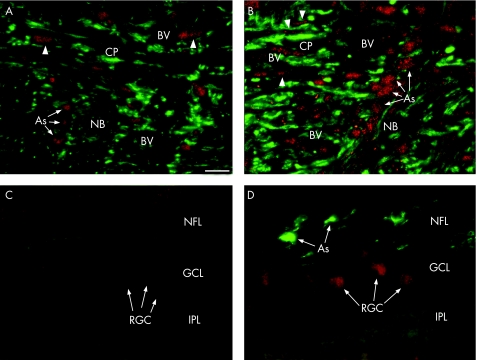
In the monkey without glaucoma, the expression of EphB1 receptor localised to the nucleus of ONH astrocytes, whereas EphB1 was barely detectable in the retina without glaucoma. In the glaucomatous monkey, EphB1 expression was enhanced in the astrocytes nuclei and cytoplasm, in perivascular cells lining the wall vessel and in GFAP‐negative lamina cribrosa cells surrounding blood vessels (fig 3A,B). In the monkey glaucomatous retina, EphB1 was highly expressed in the RGCs (fig 3C,D).
Figure 3 EphB1 expression in monkey with and without glaucomatous optic nerve and retina. Double immunofluorescence staining with glial acidic fibrillar protein (GFAP; green) and EphB1 (red) of sagittal sections (monkey number 5) showed low expression of EphB1 in the monkey without glaucoma (A) localised to astrocyte nuclei. In the glaucomatous monkey optic nerve head (B), EphB1 is enhanced in astrocytes nuclei, in the GFAP‐negative lamina cribrosa cells (arrowheads) and also in perivascular cells in the lamina cribrosa, lining the vessel walls. EphB1 expression in normal monkey retina (C) is low, but shows a strong expression in the retinal ganglion cells of the glaucomatous retina (D) (monkey number 7). Scale bar: 40 μm. As, astrocytes; BV, blood vessel; CP, cribriform plates; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; NB, nerve bundle; NFL, nerve fibre layer; OPL, outer plexiform layer; VC, vitreous cavity.
Representative micrographs were chosen to demonstrate results using immunohistochemistry, which were consistent among the experimental and control tissues from monkeys with mild and moderate glaucoma; ONH tissues from eyes with advanced glaucoma exhibited very little or no staining of astrocytes (not shown).
Cultured human astrocytes
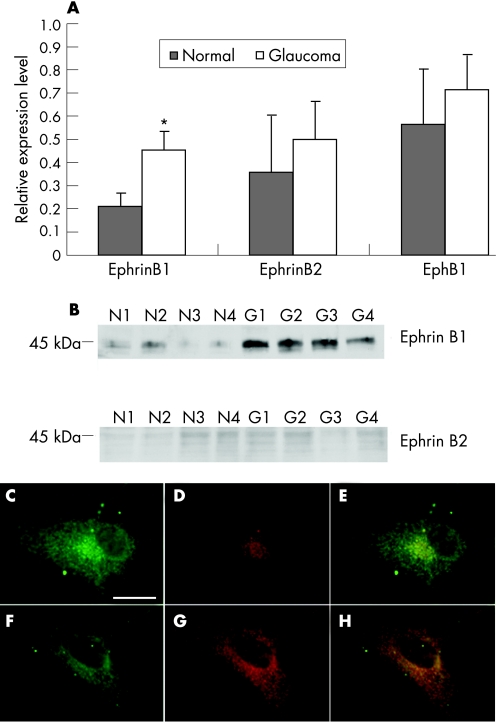
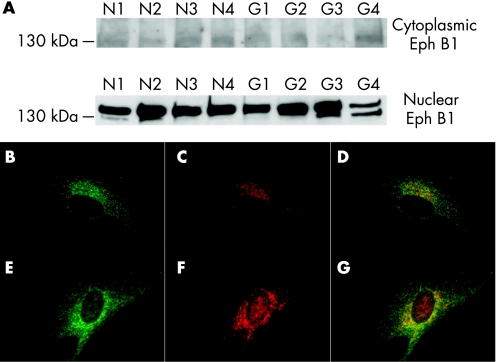
To determine whether the level of ephrinB1, ephrinB2 and EphB1 expression in cultured human astrocytes with and without glaucoma were different, we performed qRT‐PCR, western blot and immunocytochemistry. Normal astrocytes expressed low mRNA levels of ephrinB1 and ephrinB2 by qRT‐PCR. EphrinB1 expression was strongly enhanced in glaucomatous astrocytes and estimated to be two to threefold compared to astrocytes without glaucoma (p<0.005). There was no difference in the level of EphB1 mRNA between ONH astrocytes with and without glaucoma (fig 4A). Western blot analysis of cytoplasmic proteins in human astrocytes with and without glaucoma showed low expression of cytoplasmic ephrinB1 and barely detectable ephrinB2 expression. In glaucomatous astrocytes, cytoplasmic ephrinB1 expression was strongly enhanced (fig 4B) whereas ephrinB2 expression in glaucomatous ONH astrocytes remained low. Immunoblots of cytoplasmic protein showed no difference in EphB1 protein levels between cells with and without glaucoma (fig 5A). Immunoblots of nuclear protein showed nuclear EphB1 was present in normal ONH astrocytes but exhibited higher expression in glaucomatous astrocytes (fig 5A).
Figure 4 EphrinB1, ephrinB2 and EphB1 mRNA levels in human cultured astrocytes with and without glaucoma detected by qRT‐PCR. (A) Stronger expression of ephrinB1 in glaucomatous ONH astrocytes compared ONH astrocytes without glaucoma as shown by qRT‐PCR (P<0.05); ephrinB2 and EphB1 remained low in ONH astrocytes with and without glaucoma. Bars represent means and SD for cell cultures with and without glaucoma for ephrinB1, ephrinB2 and EphB1. (B) EphrinB1 is present at higher levels in glaucomatous ONH astrocytes (lanes G1–G4) compared to ONH astrocytes without glaucoma (lanes N1–N4) as shown by western blot; ephrinB2 remained low in ONH astrocytes with and without glaucoma. Double immunofluorescence shows low ephrinB1 (red) staining in (C, D, E) ONH astrocytes without glaucoma, but enhanced in the nuclear and perinuclear area and in cell membrane of glaucomatous (F, G, H) astrocytes. Scale bar: 40 μm.
Figure 5 EphB1 expression in human cultured astrocytes with and without glaucoma. (A) Representative western blot of EphB1 shows low expression in the cytoplasm of both the ONH astrocytes without glaucoma (lanes N1–N4) and with glaucoma (lanes G1–G4), but is present in the nuclei of optic nerve head (ONH) astrocytes without glaucoma and strongly enhanced in the nuclei of glaucomatous ONH astrocytes. Double immunofluorescence shows low EphB1 (red) staining in (B, C, D) ONH astrocytes with glaucoma, but enhanced in the nuclear and perinuclear area of glaucomatous (E, F, G) astrocytes. Scale bar: 40 μm. AS, astrocytes; BV, blood vessel; CP, cribriform plates; GCL, ganglion cell layer; IPL, inner plexiform layer; NB, nerve bundle; NFL, nerve fibre layer; RGC, retinal ganglion cell.
Immunostaining of ephrinB1 in human astrocytes with glaucoma in culture was low, but enhanced in nuclear and perinuclear area and in the cell membrane in astrocytes from glaucomatous eyes (fig 4C–H). EphrinB2 was barely detectable in both astrocytes with and without glaucoma (data not shown). EphB1 expression was low in ONH astrocytes without glaucoma, but enhanced in the nuclear and perinuclear area of glaucomatous astrocytes (fig 5B–D).
Discussion
The main objective of this study was to demonstrate the presence of the EphB1/ephrinB1 pathway in the glaucomatous ONH. Our findings suggest that activation of the EphB1/ephrinB1 pathway represents a recapitulation of a developmental programme, which may play a protective role during optic nerve degeneration by preserving the blood–nerve barrier and preventing invasion of non‐neural cells into areas of axonal loss, as an attempt to provide boundaries to the axonal damage.
Recent studies have demonstrated the overexpression of ephrin/Eph in injured spinal cord,21 after denervation in the mouse hippocampus30 and in multiple sclerosis lesions,31 presumably affecting the migration and adhesion of inflammatory cells to extracellular matrix and suggesting a role of ephrin/Eph in adult neural tissue repair. Expression of ephrinB proteins and the EphB receptor in vascular and perivascular cells are currently under study because they contribute to regulation of angiogenesis in cancer,32 and enhancing angiogenesis in ocular diseases such as proliferative diabetic retinopathy and retinopathy of prematurity.33
Ephrin B1 was strongly expressed in glaucomatous ONH astrocytes in vivo and in vitro, confirming previous microarray results.14 Astrocytes in the glaucomatous ONH and in the retrolaminar optic nerve exhibited abundant localisation of ephrinB1 in the cytoplasm and in the nucleus. EphrinB1‐expressing astrocytes surrounded small blood vessels in glaucomatous ONH. EphB1 was also overexpressed in perivascular cells lining the vessel wall and in lamina cribrosa cells surrounding blood vessels in the glaucomatous ONH. We hypothesise that the Eph/ephrin signalling in glaucoma plays a role preventing invasion of non‐neural cells into areas of axonal loss as an attempt to preserve the blood–nerve barrier.
In this study there was loss of ephrin signalling in advanced glaucomatous eyes compared to mild and moderate stages of damage. The loss of ephrin signalling in advanced glaucoma may explain microglia and macrophage activation34 and therefore contributes to the axonal loss in glaucomatous optic neuropathy.
Although both ephrinB ligand and receptor are transmembrane proteins and are classically described in the membrane, there is evidence that full‐length receptor–ligand complexes are internalised into the cytoplasm in EphB‐expressing cells18,35,36,37 and, then, large vesicles take the Eph‐ephrin complex to the nucleus.38 Under normal conditions this mechanism may allow sequestration of the Eph receptor in the nucleus away from the membrane‐located ephrin in quiescent ONH astrocytes; thus, preventing changes in cell position in the lamina cribrosa. Previous studies have shown that the ephrins affect immune regulation, antigen recognition, inhibition of chemotaxis,39 cytokine production40 and T cell adhesion into extracellular matrix molecules, such as fibronectin, within perivascular inflammatory cuffs and as they migrate within the parenchyma.40,41 Ephrin‐B1 and EphB receptors are upregulated in glaucoma when astroglial activation, axonal loss and ONH tissue remodelling occur. The phagocytic function of reactive astrocytes has not been explored in glaucomatous optic neuropathy, but an important role of reactive astrocytes in response to neural degeneration is the removal of axonal debris through phagocytosis.42,43 Eph/ephrin signals control repulsive and attractive interactions during tissue patterning in embryonic development and in tissue remodelling in adults. Eph/ephrin interactions result in axonal repulsion and growth cone collapse,44,45 thereby inhibiting axon branching.15 Our data suggest for the first time that Eph/ephrin interactions may be part of the mechanism that prevents axon regrowth in glaucoma, an important step in optic nerve repair.
In summary, upregulation of Eph/ephrin pathway may play a protective role by limiting axonal damage and inflammatory cell invasion. Further studies are needed to elucidate the mechanisms triggered by the Eph/ephrinB pathway activation and their relevance in glaucoma.
Abbreviations
GFAP - glial acidic fibrillar protein
IOP - intraocular pressure
NDRI - National Disease Research Interchange
ONH - optic nerve head
POAG - primary open angle glaucoma
RGC - retinal ganglion cell
Footnotes
This work was supported by NIH EY‐06416 (MRH), and EY02698 (PLK), Research to Prevent Blindness, Ocular Physiology Research and Education Foundation, and Retina Research Foundation (Watler H Helmerich Chair).
Competing interests: None.
References
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ 200482887–888. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley H A. New paradigms in the mechanisms and management of glaucoma. Eye 2004291–8. [DOI] [PubMed] [Google Scholar]
- 3.Coleman A L, Brigatti L. The glaucomas. Minerva Med 200192365–379. [PubMed] [Google Scholar]
- 4.Resnikoff S, Pascolini D, Etya'ale D.et al Global data on visual impairment in the year 2002. Bull World Health Organ 200482844–851. [PMC free article] [PubMed] [Google Scholar]
- 5. The AGIS Investigators, The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000130429–440. [DOI] [PubMed] [Google Scholar]
- 6.Kass M A, Heuer D K, Higginbotham E J.et al The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Arch Ophthalmol 2002120701–13 discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 7.Emre M, Orgul S, Gugleta K.et al Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol 200488662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez M R. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res 200019297–321. [DOI] [PubMed] [Google Scholar]
- 9.Morgan J E. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye 200014437–444. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld A H, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist 20039485–495. [DOI] [PubMed] [Google Scholar]
- 11.Tezel G, Chauhan B C, LeBlanc R P.et al Immunohistochemical assessment of the glial mitogen‐activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci 2003443025–3033. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Agapova O, Parker A.et al DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol Genomics 200417157–169. [DOI] [PubMed] [Google Scholar]
- 13.Tezel G, Hernandez M R, Wax M B. In vitro evaluation of reactive astrocyte migration, a component of tissue remodeling in glaucomatous optic nerve head. Glia 200134178–189. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez M R, Agapova O A, Yang P.et al Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia 20023845–64. [DOI] [PubMed] [Google Scholar]
- 15.Pasquale E B. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 20056 pp 462-75 Erratum in: Nat Rev Mol Cell Biol20056589. [DOI] [PubMed] [Google Scholar]
- 16.Mellitzer G, Xu Q, Wilkinson D G. Control of cell behavior by signaling through Eph receptors and ephrins. Curr Opin Neurobiol 200010400–408. [DOI] [PubMed] [Google Scholar]
- 17.Katakowski M, Zhang Z, Decarvalho A C.et al EphB2 induces proliferation and promotes a neuronal fate in adult subventricular neural precursor cells. Neurosci Lett 2005385204–209. [DOI] [PubMed] [Google Scholar]
- 18.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol 200416580–589. [DOI] [PubMed] [Google Scholar]
- 19.Oster S F, Deiner M, Birgbauer E.et al Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol 200415125–136. [DOI] [PubMed] [Google Scholar]
- 20.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol 20023475–486. [DOI] [PubMed] [Google Scholar]
- 21.Bundesen L Q, Scheel T A, Bregman B S.et al Ephrin‐B2 and EphB2 regulation of astrocyte‐meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci 2003237789–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willson C A, Miranda J D, Foster R D.et al Transection of the adult rat spinal cord upregulates EphB3 receptor and ligand expression. Cell Transplant 200312279–290. [DOI] [PubMed] [Google Scholar]
- 23.Pena J D, Agapova O, Gabelt B T.et al Increased elastin expression in astrocytes of the lamina cribrosa in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci 2001422303–2314. [PubMed] [Google Scholar]
- 24.Yucel Y H, Kalichman M W, Mizisin A P.et al Histomorphometric analysis of optic nerve changes in experimental glaucoma. J Glaucoma 1999838–45. [PubMed] [Google Scholar]
- 25.Sanchez R M, Dunkelberger G R, Quigley H A. The number and diameter distribution of axons in the monkey optic nerve. Invest Ophthalmol Vis Sci 1986271342–1350. [PubMed] [Google Scholar]
- 26.Pena J D, Netland P A, Vidal I.et al Elastosis of the lamina cribrosa in glaucomatous optic neuropathy. Exp Eye Res 199867517–524. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi S, Vidal I, Pena J D.et al Expression of neural cell adhesion molecule characterizes a subpopulation of type 1 astrocytes in human optic nerve head. Glia 199720262–273. [DOI] [PubMed] [Google Scholar]
- 28.Yang P, Agapova O, Parker A.et al DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol Genomics 200417157–169. [DOI] [PubMed] [Google Scholar]
- 29.Salvador‐Silva M, Ricard C S, Agapova O A.et al Expression of small heat shock proteins and intermediate filaments in the human optic nerve head astrocytes exposed to elevated hydrostatic pressure in vitro. J Neurosci Res 20016659–73. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ying G X, Liu X.et al Induction of ephrin‐B1 and EphB receptors during denervation‐induced plasticity in the adult mouse hippocampus. Eur J Neurosci 2005212336–2346. [DOI] [PubMed] [Google Scholar]
- 31.Sobel R A. Ephrin A receptors and ligands in lesions and normal‐appearing white matter in multiple sclerosis. Brain Pathol 20051535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murai K K, Pasquale E B. Eph receptors, ephrins, and synaptic function. Neuroscientist 200410304–314. [DOI] [PubMed] [Google Scholar]
- 33.Umeda N, Ozaki H, Hayashi H.et al Expression of ephrinB2 and its receptors on fibroproliferative membranes in ocular angiogenic diseases. Am J Ophthalmol 2004138270–279. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld A H. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol 19991171050–1056. [DOI] [PubMed] [Google Scholar]
- 35.Cowan C W, Shao Y R, Sahin M.et al Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron 200546205–217. [DOI] [PubMed] [Google Scholar]
- 36.Parker M, Roberts R, Enriquez M.et al Reverse endocytosis of transmembrane ephrin‐B ligands via a clathrin‐mediated pathway. Biochem Biophys Res Commun 200432317–23. [DOI] [PubMed] [Google Scholar]
- 37.Zimmer M, Palmer A, Kohler J.et al EphB‐ephrinB bi‐directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol 20035869–878. [DOI] [PubMed] [Google Scholar]
- 38.Marston D J, Dickinson S, Nobes C D. Rac‐dependent trans‐endocytosis of ephrinBs regulates Eph‐ephrin contact repulsion. Nat Cell Biol 20035879–888. [DOI] [PubMed] [Google Scholar]
- 39.Sharfe N, Freywald A, Toro A.et al Ephrin stimulation modulates T cell chemotaxis. Eur J Immunol 2002323745–3755. [DOI] [PubMed] [Google Scholar]
- 40.Wohlfahrt J G, Karagiannidis C, Kunzmann S.et al Ephrin‐A1 suppresses Th2 cell activation and provides a regulatory link to lung epithelial cells.J Immunol 2004172843–850. [DOI] [PubMed] [Google Scholar]
- 41.Smith L M, Walsh P T, Rudiger T.et al EphA3 is induced by CD28 and IGF‐1 and regulates cell adhesion. Exp Cell Res 2004292295–303. [DOI] [PubMed] [Google Scholar]
- 42.Bechmann I, Nitsch R. Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis‐dependent labeling technique. Glia 199720145–154. [DOI] [PubMed] [Google Scholar]
- 43.Deller T, Haas C A, Naumann T.et al Up‐regulation of astrocyte‐derived tenascin‐C correlates with neurite outgrowth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neuroscience 199781829–846. [DOI] [PubMed] [Google Scholar]
- 44.Yue Y, Su J, Cerretti D P.et al Selective inhibition of spinal cord neurite outgrowth and cell survival by the Eph family ligand ephrin‐A5. J Neurosci 19991910026–10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holland S J, Peles E, Pawson T.et al Cell‐contact‐dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase beta. Curr Opin Neurobiol 19988117–127. [DOI] [PubMed] [Google Scholar]