Retinopathy is a common side effect of interferon treatment. We report a case of symptomatic retinopathy caused by interferon α in a hypertensive patient successfully treated by management of hypertension alone and without stopping interferon α. As far as we know, this is the first reported case of interferon retinopathy successfully treated by hypertension management.
Pathogenesis of interferon retinopathy is most likely related to microcirculation damage and the impairment of angiogenesis. If interferon α therapy offers significant clinical benefit but is complicated by retinopathy, by treating other sources of microcirculation injury such as hypertension or diabetes mellitus, symptomatic retinopathy may be successfully treated without ceasing interferon α treatment.
Case report
The patient is a 55‐year‐old gentleman with multiple lung metastases from renal cell carcinoma. He had normal vision and no previous history of hypertension. In a clinical trial, he was treated with 9 MIU interferon α‐2a administered subcutaneously three times a week and 1000 mg bevacizumab intravenously every two weeks. He had excellent treatment response, with complete radiological remission of metastatic disease.
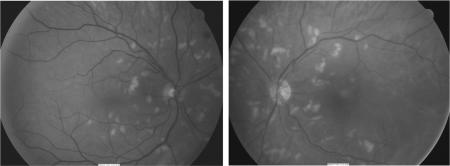
He developed blurred vision three months after starting interferon. As a side effect of bevacizumab he also became hypertensive, his blood pressure consistently 160/120 mmHg. On examination, visual acuity of his left eye was 6/6 and his right eye 6/5. Fundoscopic examination demonstrated multiple cotton wool spots in both retinas with no other signs of retinopathy (fig 1). Fluorescein angiography was unremarkable.
Figure 1 Retinal photography showing multiple cotton wool spots in both retinas before hypertension treatment.
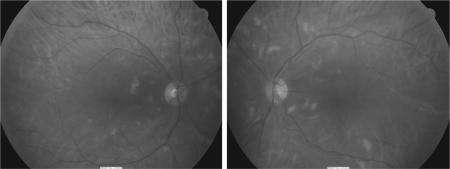
Considering the excellent cancer response, benefits of continuing interferon treatment outweighed the risk of further visual impairment. The same interferon regimen was continued with close monitoring of retinopathy. Oral anti‐hypertensive medication was started with reduction of blood pressure to 140/80. Three months later, blurred vision completely resolved, visual acuity normalized to 6/5 for both eyes and cotton‐wool spots were significantly reduced (fig 2). He continued interferon treatment with no further complications.
Figure 2 Retinal photography showing reduced cotton wool spots in both retinas after hypertension treatment.
Comment
Retinopathy, presenting with cotton wool spots, retinal haemorrhage or micro‐aneurysms on fundoscopic examination, is the most common ophthalmic side effect of interferon occurring in 24%1 of patients treated. Retinopathy rarely becomes symptomatic at viral hepatitis dosages, but at higher cancer treatment dosages there are more frequent case reports of symptomatic retinopathy.2
Pathogenesis of interferon retinopathy is still inconclusive. Ischaemia from microcirculation injury seems the most plausible explanation for retinopathy. Immune complexes deposition and inhibition of endothelial cell function, proliferation and migration have been suggested as mechanisms of microcirculation injury.3,4 Indeed interferon‐α has been shown to cause endothelial cell apoptosis in vitro.5
Reported risk factors for interferon retinopathy include hypertension,1,6 diabetes mellitus,6 high interferon dosages1 and pegylated interferon.1 Kawano found 80% of hypertensive patients treated with interferon developed retinopathy6 and d'Alteroche found hypertension carried a relative risk of 4.60 for developing retinopathy.1 Of course hypertension and diabetes also disrupt microcirculation and can each independently cause retinopathy. We postulate for that for our patient, both interferon and hypertension contributed initially to retinopathy. By reducing blood pressure, the total amount of microcirculation injury was reduced even though effects of interferon remain unchanged, thus improving retinopathy.
In patients who develop symptomatic interferon retinopathy, if benefits of interferon treatment outweigh the impact of visual impairment, interferon may be continued. Hypertension and diabetes mellitus should be optimised to reduce retinal microcirculation injury load. Retinopathy may improve once these risk factors have been optimally controlled. Retinopathy should be closely monitored for progression.
References
- 1.d'Alteroche L, Majzoub S, Lecuyer A.et al Ophthalmologic side effects during alpha‐interferon therapy for viral hepatitis. J Hepatol 20064456–61. [DOI] [PubMed] [Google Scholar]
- 2.Hejny C, Sternberg P, Lawson D H.et al Retinopathy associated with high‐dose interferon alfa‐2b therapy. Am J Ophthalmol 2001131782–787. [DOI] [PubMed] [Google Scholar]
- 3.Guyer D R, Tiedeman J, Yannuzzi L A.et al Interferon‐associated retinopathy. Arch Ophthalmol 1993111350–356. [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Ebinuma H, Nagata H.et al Interferon‐associated retinopathy in a uniform regimen of natural interferon‐alpha therapy for chronic hepatitis C. Liver 200121192–197. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser W J, Kaufman J L, Offermann M K. IFN‐alpha sensitizes human umbilical vein endothelial cells to apoptosis induced by double‐stranded RNA. J Immunol 20041721699–1710. [DOI] [PubMed] [Google Scholar]
- 6.Kawano T, Shigehira M, Uto H.et al Retinal complications during interferon therapy for chronic hepatitis C. Am J Gastroenterol 199691309–313. [PubMed] [Google Scholar]