Abstract
Aim
The pterygium represents an invasion of a wing of altered ocular surface tissue into the normal cornea. The head itself is slightly elevated and white, which is the only site of firm adhesion to the globe. The mechanisms of cell proliferation and adhesion in pterygium epithelium, however, are unknown. The aim of this study was to analyse the expression of cell adhesion molecules in pterygium tissues.
Methods
Six pterygia were surgically removed using the bare‐sclera procedure, and two normal corneas and a normal bulbar conjunctiva were also obtained. Formalin‐fixed, paraffin‐embedded tissues were analysed by immunohistochemistry with anti‐E‐cadherin and β‐catenin antibodies.
Results
Immunoreactivity for E‐cadherin was not detected in the normal cornea and conjunctiva. In contrast, all corneal and conjunctival epithelial cells showed a weak homogeneous immunoreaction for β‐catenin on the cell membrane. In the pterygium head, the thickness was relatively marked compared with the body, and normal conjunctival and corneal epithelia. E‐cadherin as well as β‐catenin was heterogeneously expressed in the cell membrane and cytoplasm of a variety of epithelial cells, whereas the expression was less marked in the body. Several epithelial cells showed intense nuclear immunoreactivity for β‐catenin. Immunoreactivity of β‐catenin, but not E‐cadherin, was detected in only a few stromal cells, which were less marked than in epithelial cells.
Conclusion
It is suggested that E‐cadherin and β‐catenin are concentrated in pterygium tissue, and are possibly involved with epithelial proliferation and adhesion.
The pterygium represents an invasion of a wing of altered ocular surface tissue into the normal cornea. It has a broad base on the nasal or temporal epibulbar surface, and a blunted apex on the cornea. The body of the pterygium can be readily lifted from the epibulbar surface. In contrast, the head itself is slightly elevated and white, which is the only site of firm adhesion to the globe.1 The mechanisms involved in corneal invasion and adhesion of the pterygium head to the globe, however, are unknown.
Recently, it has been shown that fibroblasts infiltrating the interstitium play a role in pterygium pathogenesis and development by pathobiological analyses. The modulation of fibroangiogenic growth factors and matrix‐degrading enzymes in pterygium fibroblasts contributed to the development and progression leading to corneal invasion.2,3 Histological and cytological examinations demonstrated that pterygium epithelium represented a condition of the ocular surface characterised by squamous metaplasia and goblet cell hyperplasia.4,5 We recently demonstrated that cell cycle‐related molecules played a potential role in proliferation activity in pterygium epithelial cells.4,6 These data indicate that cell proliferation and adhesion in the pterygium tissue are involved by the epithelium as well as the stroma.
Cadherins are a family of calcium‐dependent cell adhesion molecules. Through their homophilic binding interactions, cadherins play important roles in morphogenesis, morphological differentiation, and cellular proliferation.7 E‐cadherin, being the prime mediator of cell‐to‐cell adhesion, is a transmembrane glycoprotein, usually concentrated in an adhesion belt.8 β‐catenin is a multifocal protein that is both an integral component of the adherens junction which links E‐cadherin, and a pivotal member of a signal transduction pathway.9
It has recently been suggested that cell adhesion molecules are correlated with pterygium pathogenesis and development using microarray analysis.10 The aim of this study is to analyse the expression and immunolocalisation of cell adhesion molecules E‐cadherin as well as β‐catenin in normal corneal, conjunctival and pterygium tissues of humans.
Materials and methods
Preparation of human tissues
Five patients (six eyes) with primary pterygia who underwent the bare‐sclera procedure were enrolled in this study. The pterygium head and body were confirmed, and excised tissue was immediately placed on sterilised filter paper. The clinical data of the patients with pterygium are summarised in table 1. Normal corneal and bulbar conjunctival tissues were obtained from three patients. The tissues were then fixed in 4% paraformaldehyde. After fixation, slides were washed in phosphate‐buffered saline and processed for paraffin sectioning. Informed consent was obtained according to the Declaration of Helsinki. All human experiments conformed to the ethics committee in Hokkaido University Graduate School of Medicine.
Table 1 Clinical profiles in patients with primary pterygium examined in this study.
| Samples | Age | Gender | L/R | Location | Pre‐VA | Post‐VA |
|---|---|---|---|---|---|---|
| 1 | 67 | M | L | Nasally | 1.0 | 1.2 |
| 2 | 86 | F | L | Temporally | 0.2 | 0.7 |
| 3 | 53 | F | L | Nasally | 1.2 | 1.5 |
| 4 | 75 | M | R | Nasally | 0.15 | 0.2 |
| 5 | 75 | M | L | Nasally | 0.4 | 0.2 |
| 6 | 70 | M | R | Nasally | 0.5 | 0.9 |
M, male; F, female; L, left; R, right; Pre‐VA, preoperative visual acuity; Post‐VA, postoperative visual acuity.
Immunohistochemistry
Dewaxed paraffin sections were immunostained using the alkaline phosphatase complex method. Formalin‐fixed, paraffin‐embedded serial tissue sections were cut at 4 μm thickness, and endogenous peroxidase activity was inhibited by immersing the slides in 0.3% hydrogen peroxide in methanol for 30 min. As a pretreatment, microwave‐based antigen retrieval was performed in 10 mM citrate buffer (pH 6.0). Then, non‐specific binding of the primary antibody was blocked by incubating the slides in blocking serum for 20 min. The slides were serially incubated with anti‐E‐cadherin monoclonal antibody (HECD‐1, 1:200; TaKaRa Co, Japan) and anti‐β‐catenin polyclonal antibody (1:50; Cell Signaling Technology, Danvers, MA, USA), overnight at 4°C, followed by the secondary antibody. After washing, sections were incubated with a tertiary streptavidin‐alkaline phosphatase reagent (DakoCytomation, Glostrup, Denmark). The sections were again washed in phosphate‐buffered saline and incubated in Vector Red for 15 min according to the manufacturer's instructions (Vector Laboratories, Peterborough, UK), washed, and lightly counterstained with Mayer's haematoxyline for 5 seconds. Human oesophageal squamous cell carcinoma and gastric adenocarcinoma tissues served as positive controls for E‐cadherin and β‐catenin immunohistochemistry, respectively. We counted at least 100 epithelial cells in one or two microscopic fields from tissues examined. The immunopositive cells were counted by two authors (SK and MO) independently and then adjusted. Positive‐staining cells were noted by their labelling index as a percentage (%) in each specimen, and the measurements were averaged. The results of both molecules in pterygium tissues are presented as the mean (standard deviation of the mean).
Results
Normal cornea and conjunctiva
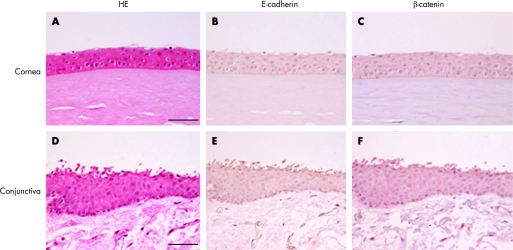
Corneal epithelium consisted of squamous epithelium without goblet cells (fig 1A). In normal bulbar conjunctiva, the epithelium consisted of several layers with round nuclei (fig 1D). A few goblet cells were intermingled in the surface. Immunoreactivity for E‐cadherin was not detected in normal corneal (fig 1B) nor conjunctival (fig 1E) epithelia. In contrast, all corneal and conjunctival epithelial cells showed a weak homogeneous immunoreaction for β‐catenin on the cell membrane (fig 1C, F), whereas the expression was not detected in Bowman's layer and the stoma. Tables 2 and 3 summarise the number of immunopositive cells for E‐cadhein and β‐catenin, respectively.
Figure 1 H&E staining (A, D) and immunodetection of E‐cadherin (B, E) and β‐catenin (C, F) in the normal human cornea (A–C) and bulbar conjunctiva (D–F). Corneal epithelium is comprised of squamous epithelium without goblet cells (A). In the normal bulbar conjunctiva, the epithelium consists of several layers with round nuclei (D). Immunoreactivity for E‐cadherin is not detected in normal corneal (B) and conjunctival (E) epithelia. All corneal (C) and conjunctival (F) epithelial cells show a homogeneously weak immunopositive reaction for β‐catenin on the cell membrane, whereas expression is not detected in Bowman's layer and the stroma. Bar = 50 μm.
Table 2 The number of E‐cadherin‐immunopositive cells in normal human corneal, conjunctival and pterygium epithelial cells.
| Total | Membrane | Cytoplasm | |
|---|---|---|---|
| Normal cornea | 0 | 0 | 0 |
| Normal conjunctiva | 0 | 0 | 0 |
| Pterygium head | 84.0 | 27.3 (24.8) | 56.7 (22.4) |
| Pterygium body | 63.4 | 16.5 (4.6) | 46.9 (20.8) |
Table 3 The number of β‐catenin‐immunopositive cells in normal human corneal, conjunctival and pterygium epithelial cells.
| Total | Membrane | Cytoplasm | Nucleus/cytoplasm | |
|---|---|---|---|---|
| Normal cornea | 100 | 100 | 0 | 0 |
| Normal conjunctiva | 100 | 100 | 0 | 0 |
| Pterygium head | 80.4 | 26.7 (11.8) | 34.0 (20.0) | 19.7 (14.7) |
| Pterygium body | 68.8 | 32.1 (21.1) | 28.2 (9.6) | 8.5 (5.6) |
Pterygium head
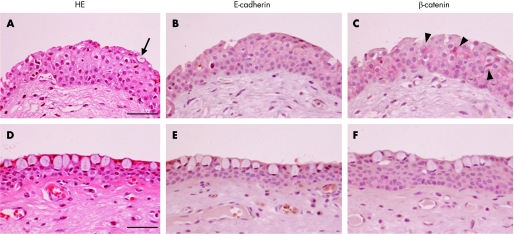
In the pterygium head, the thickness was relatively marked compared with the body, and normal conjunctival and corneal epithelia (fig 2A). The epithelium consisted of squamous metaplasia including goblet cells in the superficial layer (fig 2A, arrow). Dilated vessels and fibroblasts were found in the stroma. E‐cadherin (fig 2B) as well as β‐catenin (fig 2C) was heterogeneously expressed in a variety of epithelial cells with a membranous and cytoplasmic pattern (table 3). Several epithelial cells (19.7%) exhibited intense nuclear/cytoplasmic immunoreactivity for β‐catenin (fig 2C, arrowheads, table 3). Immunoreactivity of β‐catenin, but not E‐cadherin, was detected in only a few stromal cells, which were less marked than in epithelial cells.
Figure 2 H&E staining (A, D) and immunodetection of E‐cadherin (B, E) and β‐catenin (C, F) in the human pterygium head (A–C) and body (D–F). In the pterygium head, thickness is relatively marked compared with the body (A, D). The head epithelium consists of squamous metaplasia with several goblet cells (A, arrow) in the superficial layer. E‐cadherin (B) as well as β‐catenin (C) is predominantly expressed in a variety of epithelial cells with circumferential cytoplasmic and apical membrane patterns. Several epithelial cells show dense nuclear and cytoplasmic immunoreactivity for β‐catenin (C, arrowheads). In the pterygium body, the epithelium consists of a few layers with round cells including goblet cells (D). The thickness was relatively thin compared to the head. Immunoreactivity for E‐cadherin (E) and β‐catenin (F) is detected in the epithelium, but not in the stroma, with an apical membrane pattern. Bar = 50 μm.
Pterygium body
In the pterygium body, the epithelium consisted of a few layers with round cells including goblet cells (fig 2D). The thickness was relatively thin compared to layers of the head. Irregular degenerated elastic fibres were located in the stroma. Several epithelial cells showed immunoreactivity for E‐cadherin (fig 2E) and β‐catenin (fig 2F), with a predominantly cytoplasmic pattern, while the immunoreaction was less marked than that in the head (table 3).
Discussion
We demonstrated that E‐cadherin was expressed in a variety of epithelial cells in the pterygium head compared to the body, and was absent in the normal cornea and conjunctiva. This suggests that E‐cadherin is concentrated in the pterygium head as a pathologic state of the conjunctiva. In contrast, all corneal epithelial cells showed immunoreactivity for β‐catenin on the cell membrane. In fact, E‐cadherin, an intercellular adhesion molecule, is usually concentrated in an adhesion belt,8 and forms a complex with β‐catenin. These findings indicate that the upregulation of E‐cadherin in the pterygium head plays a potential role in adhesion to the globe.
Normal conjunctival as well as corneal epithelial cells showed a weak homogeneous immunoreaction for β‐catenin on the cell membrane. This suggests that normal corneal and conjunctival epithelia are in an unstimulated condition.11 In contrast, several epithelial cells exhibited heterogeneous intense nuclear/cytoplasmic immunoreactivity for β‐catenin in the pterygium head. The immunolocalisation indicates that β‐catenin is accumulated in the cytoplasm and is translocated to the nucleus, which is consistent with the stimulated condition.11 We recently demonstrated that proliferation activity is high in the pterygium head compared to the body, which is possibly associated with cyclin D1, a G1 cyclin involved in cell cycle progression.4 The nuclear translocation of β‐catenin is correlated with disassembly of the E‐cadherin adhesion complex, which leads to an increasing expression of cyclin D1, a β‐catenin‐T‐cell‐specific transcription factor/lymphoid enhancer‐binding factor 1 target gene.12 Taken together, epithelial proliferation in the pterygium head might be regulated by a downstream pathway in activated β‐catenin signalling.
The mechanism involved in the activation of β‐catenin has recently been indicated, which possibly contributes to the pathogenesis of human diseases. Li et al demonstrated that transforming growth factor (TGF)‐β stimulated cyclin D1 expression through the activation of β‐catenin signalling.13 In primary pterygia, it has been confirmed that TGF‐β is expressed immunohistochemically, whereas the expression is less marked in the normal conjunctiva than in the pterygium.14 This suggests that specific growth factors play a pivotal role in pterygium epithelial proliferation as upstream activators of β‐catenin signalling.
We recently reported that KL‐6, correlated with the sugar moiety of MUC‐1,15 was characteristically expressed in pterygium epithelia.6 MUC‐1 is a membrane‐associated mucin known to interfere with both cell–cell and cell–matrix adhesions. The cytoplasmic tail of MUC1 binds β‐catenin, and interaction with the E‐cadherin complex leads to the disruption of cell adhesion, which could support cell detachment and migration.16 These findings suggest that the E‐cadherin/β‐catenin complex in pterygium epithelia is directly involved via MUC‐1, which subsequently contributes to migration to the cornea.
Acknowledgements
This study was supported by a grant for Research on Sensory and Communicative Disorders from The Ministry of Health, Labor, and Welfare, and by Grants‐in‐aid for Scientific Research from The Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
Footnotes
Competing interests: None declared.
References
- 1.Kaufman H, Barron B, McDonald M.et alThe cornea. Churchill Livingstone 1988
- 2.Solomon A, Grueterich M, Li D Q.et al Overexpression of insulin‐like growth factor‐binding protein‐2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci 200344573–580. [DOI] [PubMed] [Google Scholar]
- 3.Dushku N, John M K, Schultz G S.et al Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol 2001119695–706. [DOI] [PubMed] [Google Scholar]
- 4.Kase S, Takahashi S, Sato I.et al Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br J Ophthalmol 200791958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan C M, Liu Y P, Tan D T. Ocular surface changes in pterygium. Cornea 20022138–42. [DOI] [PubMed] [Google Scholar]
- 6.Kase S, Kitaichi N, Furudate N.et al Increased expression of mucinous glycoprotein KL‐6 in human pterygium. Br J Ophthalmol 2006901208–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington K J, Syrigos K N. The role of E‐cadherin‐catenin complex: more than an intercellular glue? Ann Surg Oncol 20007783–788. [DOI] [PubMed] [Google Scholar]
- 8.Takeichi M. The cadherins: cell‐cell adhesion molecules controlling animal morphogenesis. Development 1988102639–655. [DOI] [PubMed] [Google Scholar]
- 9.Bullions L C, Levine A J. The role of beta‐catenin in cell adhesion, signal transduction, and cancer. Curr Opin Oncol 19981081–87. [DOI] [PubMed] [Google Scholar]
- 10.John‐Aryankalayil M, Dushku N, Jaworski C J.et al Microarray and protein analysis of human pterygium. Mol Vis 20061255–64. [PubMed] [Google Scholar]
- 11.Gordon M D, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 200628122429–22433. [DOI] [PubMed] [Google Scholar]
- 12.Koenig A, Mueller C, Hasel C.et al Collagen type I induces disruption of E‐cadherin‐mediated cell‐cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res 2006664662–4671. [DOI] [PubMed] [Google Scholar]
- 13.Li T F, Chen D, Wu Q.et al Transforming growth factor‐beta stimulates cyclin D1 expression through activation of beta‐catenin signaling in chondrocytes. J Biol Chem 200628121296–21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor‐beta and tumor necrosis factor‐alpha in the pterygium. Acta Histochem 199698195–201. [DOI] [PubMed] [Google Scholar]
- 15.Kohno N, Kyoizumi S, Tanabe M.et al Detection of a circulating tumor‐associated antigen with a murine monoclonal antibody, LISA 101, selected by reversed indirect enzyme‐linked immunosorbent assay. Cancer Res 1989493412–3419. [PubMed] [Google Scholar]
- 16.Leroy X, Aubert S, Ballereau C.et al Diffuse expression of MUC 1 in metastases of renal clear cell carcinoma as a possible therapeutic target for renal cancer. Histopathology 200547435–436. [DOI] [PubMed] [Google Scholar]