Abstract
Background/aims
There are several animal studies to suggest that pigment epithelium‐derived factor (PEDF) may exert beneficial effects on diabetic retinopathy and uveitis by acting as an endogenous antioxidant. However, the interrelationship between PEDF and total antioxidant capacity in the human eye remains to be elucidated. In this study, PEDF and total antioxidant levels were determined in the aqueous humour of patients with proliferative diabetic retinopathy (PDR) and uveitis, and the relationship between these two markers was investigated.
Methods
Aqueous humour levels of PEDF and total antioxidant capacity were determined by an ELISA system in 34 uveitis and 9 PDR samples.
Results
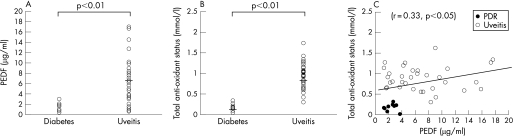
Aqueous humour levels of PEDF and total antioxidant capacity were significantly lower in patients with PDR than those with uveitis (1.8±0.2 μg/ml vs 6.4±0.8 μg/ml and 0.17±0.03 mmol/l vs 0.85±0.05 mmol/l, respectively, p<0.01). A positive correlation between PEDF and total antioxidant capacity was found in patients with PDR and uveitis (r = 0.33, p<0.05).
Conclusion
This study demonstrated that PEDF levels were associated with total antioxidant capacity in aqueous humour levels in humans. These observations suggest that substitution of PEDF may be a therapeutic target for oxidative stress‐involved eye diseases, especially PDR.
Pigment epithelium‐derived factor (PEDF) is a glycoprotein that belongs to the superfamily of serine protease inhibitors.1 It was first purified from the conditioned media of human retinal pigment epithelial cells with neuronal differentiating activity.1 Recently, PEDF has been shown to be the most potent inhibitor of angiogenesis in cell culture and animal models; it inhibited retinal endothelial cell (EC) growth and migration, and suppressed ischaemia‐induced retinal neovascularisation.2 Further, there are several animal studies to suggest that PEDF may exert beneficial effects on diabetic retinopathy and uveitis by acting as an endogenous antioxidant.3,4,5,6 Indeed, administration of PEDF prevents diabetes‐elicited or advanced glycation end products (AGE)‐elicited retinal leukostasis, an initial step of early diabetic retinopathy.4 PEDF inhibits the AGE‐induced vascular hyperpermeability and angiogenesis by blocking vascular endothelial growth factor (VEGF) signalling as well.5 In addition, PEDF decreases retinal levels of pro‐inflammatory cytokines in experimental diabetes, thus acting as an endogenous anti‐inflammatory agent.6 PEDF has also been shown recently to inhibit lipopolysaccharide‐driven macrophage activation in vitro and in vivo.7
PEDF levels in aqueous humour or vitreous are decreased in patients with diabetes, especially with PDR, suggesting that a loss of PEDF in the human eye may contribute to the development and progression of PDR.8,9,10 In contrast, in PDR, we have found that aqueous humour levels of PEDF are elevated rather than decreased in patients with active uveitis and are correlated with those of inflammatory biomarkers such as TNFα and monocyte chemoattractant protein‐1.11,12 These findings suggest that PEDF aqueous humour levels may be elevated as a counter‐system against inflammation or oxidative stress in patients with uveitis and may be a novel biomarker for the activity of uveitis. Accordingly, although animal studies suggest the potential utility of PEDF administration for the treatment of PDR and uveitis,3,4,5,6 the kinetics and pathophysiological role of PEDF in aqueous humour may differ between these disorders. Therefore, whether PEDF aqueous humour levels could reflect endogenous antioxidant capacity in the eye of PDR and uveitis remains to be elucidated. In this study, we determined PEDF and total antioxidant levels in the aqueous humour of patients with PDR and uveitis, and investigated the relationship between these markers.
Patients and methods
This study involved 9 patients with PDR (5 men and 4 women) with a mean age of 57.7 (SD 2.2) years old and 34 age‐matched and sex‐matched patients with uveitis. All patients with PDR received panretinal photocoagulation and their known duration of diabetes was 9.1 (SD 3.0) years and current level of HbA1c was 6.9±1.3% (mean±SE). A diagnosis of diabetes was made by the criteria of the ADA reported in 1997. Patients with various clinical entities of strictly diagnosed active uveitis with a mean age of 48.5 (SD 3.0) years (n = 34, 16 men and 18 women; 13 infectious uveitis and 21 non‐infectious uveitis) were also included. Informed consent was obtained from all patients. Aqueous humour was collected from patients under aseptic conditions. PEDF aqueous humour levels and total antioxidant capacity were measured as described previously.13,14,15 Inter‐assay (n = 17) and intra‐assay (n = 14) coefficient of variations of PEDF ELISA were 4.7% and 7.3%, respectively.13,14 Recovery of the added recombinant PEDF in serum samples was 94.2±1.7% (mean ± SD). The assay linearity was shown intact with serial dilution of serum. We also confirmed the specific interaction between the PEDF antibody used for ELISA and PEDF in the samples with western blot analysis. Data were analysed by the Mann–Whitney U test and Pearson's correlation coefficient by rank test. p<0.05 was considered to be significant.
Results
As shown in fig 1A and 1B, mean aqueous humour levels of PEDF and total antioxidant capacity were significantly lower in patients with PDR than in patients with uveitis (1.8±0.2 μg/ml vs 6.4±0.8 μg/ml and 0.17±0.03 mmol/l vs 0.85±0.05 mmol/l, respectively, p<0.01). A positive correlation between PEDF and total antioxidant capacity was found in patients with PDR and uveitis (r = 0.33, p<0.05).
Figure 1 Aqueous humour levels of PEDF (A) and total antioxidant capacity (B) in patients with PDR and uveitis. The horizontal line indicates the mean concentration. Mann–Whitney U test. (C) Correlation between the aqueous humour levels of PEDF and total antioxidant capacity in patients with PDR and uveitis. Pearson's correlation coefficient by rank test.
Discussion
The present results demonstrated for the first time that the aqueous humour levels of PEDF and total antioxidant capacity were significantly decreased in patients with PDR, compared with age‐matched and sex‐matched patients with active uveitis. Further, we showed here that PEDF levels in aqueous humour were positively correlated with total antioxidant capacity in the eye of PDR and uveitis. The present results indicate that PEDF may act as an endogenous antioxidative agent in various eye diseases in humans. There are several animal studies to suggest that PEDF may exert beneficial effects on the progression of diabetic retinopathy and uveitis mainly through its antioxidative properties.3,4,5,6 Indeed, PEDF could inhibit retinal leukostasis, inflammation, hyperpermeability and angiogenesis, key steps of diabetic retinopathy in animal models, by blocking NADPH oxidase‐mediated reactive oxygen species generation.3,4,5,6,16,17 Further, PEDF has been shown recently to prevent retinal inflammation by suppressing macrophage activation in vivo.7 Taken together, our present observations suggest that substitution of PEDF may be a novel therapeutic target for oxidative stress‐involved eye disease in humans, especially PDR.
Abbreviations
AGE - advanced glycation end products
EC - endothelial cell
PDR - proliferative diabetic retinopathy
PEDF - pigment epithelium‐derived factor
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None declared.
References
- 1.Tombran‐Tink J, Chader C G, Johnson L V. PEDF: Pigment epithelium‐derived factor with potent neuronal differentiative activity. Exp Eye Res 199153411–414. [DOI] [PubMed] [Google Scholar]
- 2.Duh E J, Yang H S, Suzuma I.et al Pigment epithelium‐derived factor suppresses ischemia‐induced retinal neovascularization and VEGF‐induced migration and growth. Invest Ophthalmol Vis Sci 200243821–829. [PubMed] [Google Scholar]
- 3.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005112279–2299. [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi S, Matsui T, Nakamura K.et al Pigment epithelium‐derived factor (PEDF) prevents diabetes‐ or advanced glycation end products (AGE)‐elicited retinal leukostasis. Microvasc Res 20067286–90. [DOI] [PubMed] [Google Scholar]
- 5.Yamagishi S, Nakamura K, Matsui T.et al Pigment epithelium‐derived factor inhibits advanced glycation end product‐induced retinal vascular hyperpermeability by blocking reactive oxygen species‐mediated vascular endothelial growth factor expression. J Biol Chem 200628120213–20220. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S X, Wang J J, Gao G.et al Pigment epithelium‐derived factor (PEDF) is an endogenous anti‐inflammatory factor. FASEB J 200620323–325. [DOI] [PubMed] [Google Scholar]
- 7.Zamiri P, Masli S, Streilein J W.et al Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci 2006473912–3918. [DOI] [PubMed] [Google Scholar]
- 8.Spranger J, Osterhoff M, Reimann M.et al Loss of the antiangiogenic pigment epithelium‐derived factor in patients with angiogenic eye disease. Diabetes 2002502641–2645. [DOI] [PubMed] [Google Scholar]
- 9.Ogata N, Tombran‐Tink J, Nishikawa M.et al Pigment epithelium‐derived factor in the vitreous is low in diabetic retinopathy and high in rhegmatogenous retinal detachment. Am J Ophthalmol 2001132378–382. [DOI] [PubMed] [Google Scholar]
- 10.Boehm B O, Lang G, Volpert O.et al Low content of the natural ocular anti‐angiogenic agent pigment epithelium‐derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia 200346394–400. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y, Yamagishi S, Matsui T.et al Increased levels of pigment epithelium‐derived factor (PEDF) in aqueous humor of patients with uveitis. Br J Ophthalmol 200791149–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida Y, Yamagishi S, Matsui T.et al Positive correlation between pigment epithelium‐derived factor and monocyte chemoattractant protein‐1 levels in the aqueous humour of patients with uveitis. Br J Ophthalmol 200791737–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamagishi S, Adachi H, Abe A.et al Elevated serum levels of pigment epithelium‐derived factor (PEDF) in the metabolic syndrome. J Clin Endocrimol Metab 2006912447–2450. [DOI] [PubMed] [Google Scholar]
- 14.Motomiya Y, Yamagishi S, Adachi H.et al Increased serum concentrations of pigment epithelium‐derived factor in patients with end‐stage renal disease. Clin Chem 2006521970–1971. [DOI] [PubMed] [Google Scholar]
- 15.Yokoi M, Yamagishi S I, Takeuchi M.et al Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. Br J Ophthalmol 200589673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamagishi S, Matsui T, Nakamura K.et al Pigment‐epithelium‐derived factor (PEDF) inhibits angiotensin‐II‐induced vascular endothelial growth factor (VEGF) expression in MOLT‐3 T cells through anti‐oxidative properties. Microvasc Res 200671222–226. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S, Nakamura K, Ueda S.et al Pigment epithelium‐derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti‐oxidative mechanism of PEDF. Cell Tissue Res 2005320437–445. [DOI] [PubMed] [Google Scholar]