Abstract
Aim
Intravitreal injection of anti‐vascular endothelial growth factor (VEGF) antibody (bevacizumab, Avastin) has become one of the chief choices for the treatment of macular oedema and neovascular age‐related macular degeneration. However, the effect of blocking the VEGF function has not been thoroughly explored in vivo. A previous study has reported that intravitreal injection of bevacizumab had no retinal toxicity on rats; however, bevacizumab is human‐specific and does not react with rat VEGF. In this study, the authors examined the effect of anti‐rat VEGF antibody and bevacizumab on rat retina in vivo and in vitro, especially focusing on retinal ganglion cells (RGCs).
Methods
In vitro, rat RGCs were purified by a two‐step immunopanning procedure, and incubated in the presence of VEGF, bevacizumab, anti‐rat VEGF antibody, and control‐IgG for three days. The number of viable RGCs was counted. In vivo, after intravitreal injections of bevacizumab, anti‐rat VEGF antibody, and control‐IgG, viable RGCs were visualised by retrolabelling with Fluo‐gold and enumerated to examine the toxicity.
Results
In vivo, the mean (standard deviation) number of viable RGCs in the VEGF‐treated group (0.99 (0.29) vs control), the bevacizumab‐treated group (1.0 (0.23) vs control), the anti‐rat VEGF antibody‐treated group (0.98 (0.18) vs control) and the control IgG‐treated group (0.98 (0.19) vs control) was not statistically different from that of the control group after 3 days. In vitro, the mean (SD) number of viable RGCs in the bevacizumab‐treated group (2613 (230)/mm2), the anti‐rat VEGF antibody‐treated group (2600 (140)/mm2) and the control IgG‐treated group (2656 (150)/mm2) was not statistically different from that of the control group (2656 (150)/mm2) after 7 days. There were no apparent histological abnormalities.
Conclusion
This study suggests that bevacizumab and anti‐rat VEGF antibody have no short‐term, direct retinal toxicity using the rat model. Intravitreal injection of bevacizumab shows no short‐term, direct toxicity on RGCs.
Vascular endothelial growth factor (VEGF) has been implicated as the key angiogenic stimulus responsible for the formation of choroidal neovascularisation in age‐related macular degeneration (AMD).1
Recently, bevacizumab (Avastin; Genentech Inc, San Francisco, CA, USA), an antibody that binds human VEGF with high affinity, was approved for treating colorectal cancer patients.2 It is a humanised monoclonal antibody that binds all isoforms of VEGF and interferes with its binding to receptors, thus inhibiting its signal. It has been demonstrated that intravitreous injection of bevacizumab is effective for patients with neovascular AMD, improving visual acuity and reducing retinal oedema.3,4 Bevacizumab is currently being used at multiple centres in the USA, Europe and Japan for the treatment of neovascular AMD. Clinically, to date, no retinal toxicity has been reported after intravitreal injection of bevacizumab, but limited safety data are available.
Previous groups have evaluated the safety of intravitreal injection of bevacizumab in rabbits using electrophysiological testing and histopathological analysis.5 Another group has reported that bevacizumab could exert a moderate growth inhibition on pig choroidal endothelial cells and that high dose bevacizumab may be harmful to a human retinal pigment epithelial cell line, ARPE‐19 cells, in vitro.6 Some groups have also reported the safety of bevacizumab on retina with studies using murine cells.7 However, it should be noted that bevacizumab is specific to human VEGF.8 As has been clarified by structural analysis, bevacizumab does not bind with murine VEGF8 because of an amino acid substitution in the bevacizumab‐binding site.
Vascular endothelial growth factor exerts neuroprotective effects on central nervous system. For example, VEGF controls the correct migration of facial branchiomotor neurons in the developing hindbrain and stimulates the proliferation of neural stem cells in enriched environments and after cerebral ischaemia in vivo.9 Reduced levels of VEGF have been also implicated in a polyglutamine‐induced model of motor neuron degeneration.10 Similar neuroprotective effects of VEGF have been described for axotomised retinal ganglion cells in vivo.11 This may raise the concern that therapeutic inhibition on VEGF for the treatment of neovascular eye diseases may cause neuronal damage even though any clinical evidence for this theoretical assumption is lacking to date.
In this study, to determine the potential toxicity of intravitreal bevacizumab and the inhibition of VEGF signalling, we used anti‐rat VEGF antibody, or bevacizumab in Wister rats and evaluated their toxicity to retinal layers, and in particular to retinal ganglion cells (RGCs) both in vivo and in vitro.
Materials and methods
Animals
Wister rats (6–8 weeks and 8 days old) were purchased from Saitama Laboratory Animal Supply Inc (Saitama, Japan). The animals were kept under standard laboratory conditions with a 12‐h light‐dark cycle. All experiments were conducted in accordance with the Animal Care and Use Committee and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Western blot analysis
The eye balls of Wister rats (6 weeks old) were enucleated. The tissues of retinas and choroids were resuspended in RIPA buffer and the lysates were incubated on ice for 20 min and then centrifuged at 15 000 rpm to remove cellular debris. Ten μl of protein extracts were electrophoresed on 10% SDS‐PAGE gels. Proteins were transferred to Hybond nitrocellulose membranes for 1 h at 14 V in Tris‐based buffer. Non‐specific binding sites were blocked with 5% non‐fat dried milk for 1 h and membranes incubated for 1 h at RT in bevacizumab (0.25 mg/ml) and anti‐rat VEGF antibody (0.1 μg/ml; R&D Systems, Minneapolis, USA). Antibody‐treated membranes were visualised by the ECL Western Blotting Analysis System (Amersham Biosiences, Buckinghamshire, UK) with rabbit anti‐mouse IgG (Amersham Biosiences) and anti‐goat IgG.
Purification and culture of RGCs
Retinal ganglion cells were purified by a two‐step immunopanning procedure, as described previously.12,13 Briefly, the dissociated cells of retinas from 6‐day‐old Wister rats were incubated in flasks (Nunc A/S, Roskilde, Denmark) coated with an anti‐rat macrophage monoclonal antibody (1:50; Chemicon, Temecula, CA, USA) to exclude macrophages, and then incubated in tubes (Corning, Acton, MA, USA) coated with an anti‐rat Thy1.1 monoclonal antibody (1:300; Chemicon). RGCs adherent to the tubes were collected by centrifugation at 600 rpm for 5 min and seeded on 13‐mm glass coverslips in a 24‐well plate that had been coated with 50 μg/ml poly‐L‐lysine (Sigma‐Aldrich, St Louis, MO, USA) and 1 μg/ml laminin (Invitrogen, Carlsbad, CA). Purified RGCs were plated at a density of approximately 1000 cells/well. RGCs were cultured in serum‐free B27 complete medium containing neurobasal medium (Invitrogen) with 1 mM L‐glutamine (Sigma‐Aldrich), B27 supplement (Invitrogen), 40 ng/ml human recombinant brain‐derived neurotropic factor (BDNF; Sigma‐Aldrich), 40 ng/ml rat recombinant ciliary neurotropic factor (CNTF; Peprotech, Rocky Hill, NJ, USA), 10 μM forskolin (Sigma‐Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin.14 To examine the effect of bevacizumab and anti‐rat VEGF antibody, RGCs were cultured for three days in 400 μl B27 complete medium containing either VEGF (10 ng/ml; R&D Systems), bevacizumab (0.25 mg/ml), anti‐rat VEGF antibody (1 μg/ml) and control rat IgG (11 μg/ml; R&D Systems). The amount of the rat anti‐VEGF antibody was determined through our results of angiogenesis assay using HUVEC cells in vitro and the antibody concentration that completely blocks the action of VEGF was employed. Plates were incubated in a tissue culture incubator with a humidified atmosphere containing 5% CO2 and 95% air at 37°C. Three days after the experiments, cell viability was determined using the fluorescence viability agent calcein‐acetoxymethyl ester (calcein‐AM, 1 μM; Molecular Probes, Eugene, OR). In the present study, a surviving RGC was defined as a cell with calcein‐AM‐stained cell body and a neurite outgrowth of at least two cell diameters from the cell body. All surviving RGCs on each glass coverslip were counted at 200× magnification under an inverted fluorescence microscope. In B27 complete medium, approximately 500 cells were counted per well. The number of RGCs after three days of culture in B27 complete medium was set at 100%. The fluorescent images of the stained RGCs were captured by a laser scanning microscope (Fluoview; Olympus, Tokyo, Japan).
Intravitreous injection
Bevacizumab, anti‐rat VEGF antibody, and control rat IgG were injected to the vitreous cavity of right globes, as has been previously described.13 General anaesthesia was induced with an intraperitoneal injection (1000 μl/kg) of a mixture (5:1) of ketamine hydrochloride (Ketalar; Sankyo, Tokyo, Japan) and xylazine hydrochloride (Celactal; Bayer, Tokyo, Japan). After mydriasis was achieved with a drop of 0.5% tropicamide, a 30‐gauge needle was inserted into the midvitreous of the right eye, guided by a stereoscopic microscope, with care taken to avoid lens and retinal injury. A single injection of 4 μl bevacizumab (3.75 mg/ml), anti‐rat VEGF antibody (15 μg/ml) and control rat IgG (15 μg/ml) was completed in one minute. For control, 4 μl of physiological saline was injected intravitreously to the left eyes of the same animals.
Retinal ganglion cell counting
Retinal ganglion cell counting was performed as has been described.13,15 Three days after the intravitreous injection, anaesthetised rats were placed in a stereotactic frame, two holes made in the skull, and 0.2 μl of 5% Fluoro‐Gold (Wako, Tokyo, Japan) in saline was injected into the superior colliculi of both sides. Each injection was made over two minutes with a syringe (Hamilton, Reno, NV, USA). Seven days thereafter, the globes were enucleated, and cornea, lens and vitreous removed. Six radial cuts were made at the peripheral retina to the equator, and the retina was separated and mounted on a slide with the vitreous side facing up. The number of cell bodies of RGCs was counted. Counts were taken from six circumferential points 1 mm eccentric from the optic nerve of the retinal flat preparation. The counts were averaged to give the count in one eye. RGC counting was performed with the observer masked as to treatment.
Histological analysis
Ten days after the intravitreous injection, the eyes were enucleated, and embedded in paraffin blocks and a 6 μm semi‐thin section was made. The sections were mounted on glass slides and stained with H&E.
Statistics
The Mann–Whitney test was used for statistical analysis. A p value of <0.05 was considered statistically significant.
Results
Anti‐rat VEGF antibody binds to rat VEGF120, VEGF144 and VEGF164
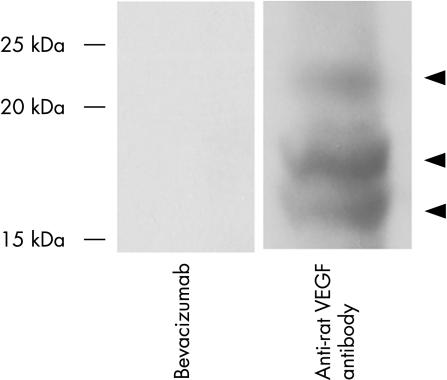
To confirm whether bevacizumab and the rat retinal antibody that we used bind rat VEGF, rat retinal and choroidal proteins were analysed by western blotting. Figure 1 shows a representative blot. Anti‐rat VEGF antibody reacted with the 29 kDa, 23 kDa and 18 kDa proteins consistent with the size of VEGF 164, VEGF 144 and VEGF 120 isoforms, whereas no bands were detected in the bevacizumab‐treated lane, confirming that bevacizumab did not bind rat VEGF and anti‐VEGF antibody used in the current study reacts with rat VEGF120, VEGF144 and VEGF164.
Figure 1 The anti‐rat vascular endothelial growth factor (VEGF) antibody used in the current study binds rat VEGF. Protein extracted from the retina and choroid of rats were analysed by western blotting using bevacizumab and anti‐rat VEGF antibody. The anti‐rat VEGF antibody reacted with the 29 kDa, 23 kDa and 18 kDa protein consistent with the size of VEGF 164, VEGF 144 and VEGF 120 isoforms (arrowheads), whereas no bands were seen in the bevacizumab‐treated lane.
Effect of bevacizumab and anti‐rat VEGF antibody on purified RGCs in vitro
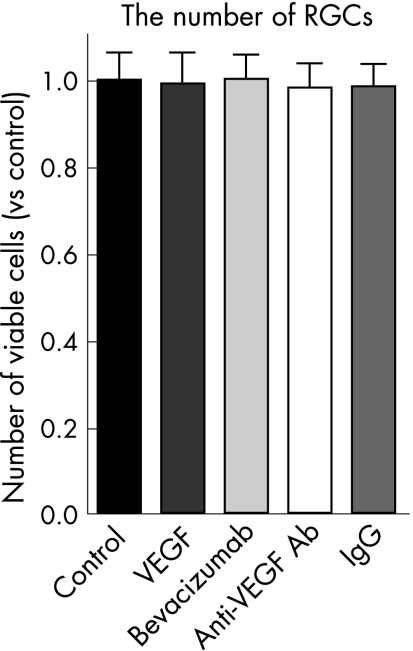
The number of viable RGCs was not affected after exposure to VEGF (the ratio of the number of viable RGCs was 0.99 (SD 0.29) compared with the non‐treated control; n = 5), bevacizumab (the ratio of the number of viable RGCs was 1.0 (0.23) compared with the non‐treated control; n = 5), anti‐rat VEGF antibody (the ratio of the number of viable RGCs was 0.98 (0.18) compared with the non‐treated control; n = 5), or control IgG (the ratio of the number of viable RGCs was 0.98 (0.19) compared with the non‐treated control; n = 5) for three days (fig 2).
Figure 2 Effect of bevacizumab and anti‐rat vascular endothelial growth factor (VEGF) antibody on purified retinal ganglion cells (RGCs) in vitro. RGCs were cultured in B27 complete medium containing VEGF (10 ng/ml), bevacizumab (0.25 mg/ml), anti‐rat VEGF antibody (1 μg/ml) and control rat IgG (1 μg/ml). Surviving RGCs were counted three days after culture and the number set at 100%. These experiments were repeated three times in two separate wells. Data are the mean (standard deviation) (n = 5). The number of viable RGCs was not affected after exposure to VEGF, bevacizumab, anti‐rat VEGF antibody or control IgG.
Effect of bevacizumab and anti‐rat VEGF antibody on rat RGCs in vivo
The numbers of viable RGCs in the bevacizumab‐treated group (2613 (230)/mm2), anti‐rat VEGF antibody‐treated group (2600 (140)/mm2) and control IgG‐treated group (2656 (150)/mm2) were not statically different from that of control group (2656 (150)/mm2) (fig 3).
Figure 3 Effect of bevacizumab and anti‐rat vascular endothelial growth factor (VEGF) antibody on retinal ganglion cells (RGCs) in vivo. The number of RGCs was counted 10 days after intravitreous injection of bevacizumab, anti‐rat VEGF antibody or control rat IgG. Three days after Fluogold was injected into the superior colliculi, the number of viable RGCs was counted from six circumferential points 1 mm eccentric from the optic nerve of the retinal flat preparation. Note that the number of RGCs was not significantly decreased in each group. The data represent the mean of results from five eyes; error bars, SEM.
Histological analysis
There were no apparent histological abnormalities in any group (fig 4).
Figure 4 Histological evaluation. Ten days after the intravitreous injection, the eyes were enucleated, and embedded in paraffin blocks and 6 μm semi‐thin section was made. The sections were mounted on glass slides and stained with H&E. There were no apparent abnormalities in any group.
Discussion
This is the first study that the authors are aware of that investigates the effects of bevacizumab and anti‐rat VEGF antibody on RGCs both in vivo and in vitro. With the same assay as was used in the current study, it has been demonstrated that intravitreal injection of indocyanine green was toxic to RGCs even in the absence of histological and electrophysiological abnormalities.13 Subsequent clinical studies support that ICG may exert toxic effects on RGCs.16,17 Thus, even in the absence of apparent abnormalities by histological as well as electrophysiological analysis, toxicity tests on RGCs are important to establish the safety of the drug for intravitreal injection. A previous study reported that bevacizumab could penetrate the retina layer but most of it remained at the inner limiting membrane and in the RGCs.18 Together with the fact that VEGF exerts neuroprotective effects on various cultured neurons of the central nervous system, this result adds importance to the investigation of the effect of bevacizumab on RGCs. With the same experimental procedure under which the toxicity of ICG was clearly demonstrated,13 the current results showed that bevacizumab and anti‐rat VEGF antibody had no retinal toxicity using a rat model in vitro and in vivo at the dose that completely blocks the biological function of VEGF, suggesting that acute toxic effects of bevacizomab on RGCs may be negligible.
Thus the present study supports that a single intravitreous injection of bevacizumab shows no toxicity on RGCs, but it remains unknown whether repeated injection of bemacizumab with a longer follow‐up would demonstrate toxicity on RGCs clinically. The half‐life of bevacizumab in eyes should be investigated and the blood kinetics after intravitreal injection should be fully researched, considering the risk of the systemic adverse effect. Future studies need to determine the appropriate frequency of intravitreous injection of bevacizumab as a treatment for AMD.
Abbreviations
AMD - age‐related macular degeneration
RGC - retinal ganglion cells
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None declared.
References
- 1.Adamis A P, Shima D T. The role of vascular endothelial growth factor in ocular health and disease. Retina 200525111–118. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W.et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 20043502335–2342. [DOI] [PubMed] [Google Scholar]
- 3.Yoganathan P, Deramo V A, Lai J C.et al Visual improvement following intravitreal bevacizumab (Avastin) in exudative age‐related macular degeneration. Retina 200626994–998. [DOI] [PubMed] [Google Scholar]
- 4.Rich R M, Rosenfeld P J, Puliafito C A.et al Short‐term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Retina 200626495–511. [DOI] [PubMed] [Google Scholar]
- 5.Feiner L, Barr E E, Shui Y B.et al Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina 200626882–888. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer M S, Wallenfels‐Thilo B, Sierra A.et al Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol 2006901316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luthra S, Narayanan R, Marques L E.et al Evaluation of in vitro effects of bevacizumab (Avastin) on retinal pigment epithelial, neurosensory retinal, and microvascular endothelial cells. Retina 200626512–518. [DOI] [PubMed] [Google Scholar]
- 8.Fuh G, Wu P, Liang W C.et al Structure‐function studies of two synthetic anti‐vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J Biol Chem 20062816625–6631. [DOI] [PubMed] [Google Scholar]
- 9.Lambrechts D, Carmeliet P. VEGF at the neurovascular interface: therapeutic implications for motor neuron disease. Biochim Biophys Acta 200617621109–1121. [DOI] [PubMed] [Google Scholar]
- 10.Sopher B L, Thomas P S, Jr, LaFevre‐Bernt M A.et al Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron 200441687–699. [DOI] [PubMed] [Google Scholar]
- 11.Kilic U, Kilic E, Jarve A.et al Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK‐1/2 and Akt pathways. J Neurosci 20062612439–12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otori Y, Wei J Y, Barnstable C J. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci 199839972–981. [PubMed] [Google Scholar]
- 13.Iriyama A, Uchida S, Yanagi Y.et al Effects of indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis Sci 200445943–947. [DOI] [PubMed] [Google Scholar]
- 14.Brewer G J, Torricelli J R, Evege E K.et al Optimized survival of hippocampal neurons in B27‐supplemented Neurobasal, a new serum‐free medium combination. J Neurosci Res 199335567–576. [DOI] [PubMed] [Google Scholar]
- 15.Mizuno K, Koide T, Yoshimura M.et al Neuroprotective effect and intraocular penetration of nipradilol, a beta‐blocker with nitric oxide donative action. Invest Ophthalmol Vis Sci 200142688–694. [PubMed] [Google Scholar]
- 16.Enaida H, Sakamoto T, Hisatomi T.et al Morphological and functional damage of the retina caused by intravitreous indocyanine green in rat eyes. Graefes Arch Clin Exp Ophthalmol 2002240209–213. [DOI] [PubMed] [Google Scholar]
- 17.Ueno S, Kondo M, Piao C H.et al Selective amplitude reduction of the PhNR after macular hole surgery: ganglion cell damage related to ICG‐assisted ILM peeling and gas tamponade. Invest Ophthalmol Vis Sci 2006473545–3549. [DOI] [PubMed] [Google Scholar]
- 18.Shahar J, Avery R L, Heilweil G.et al Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina 200626262–269. [DOI] [PubMed] [Google Scholar]