Abstract
Research has previously shown that, without methionine supplements, neural tube proteins of rat embryos cultured on bovine sera were hypomethylated and neural tubes failed to close. In the present study, to identify the proteins that became methylated during neurulation, rat embryos were first cultured on methionine-deficient bovine serum for 40 hr, then incubated with puromycin for 1 hr, and, finally, incubated with [methyl-14C]methionine and puromycin for 5 hr. On the basis of molecular weights, isoelectric points, and Western immunoblots, the methyl-14C-labeled proteins were identified as actin, αβ-tubulin, and neurofilament L. Indirect immunofluorescence studies indicated that without the addition of methionine to the culture, localization of actin and αβ-tubulin in the basal cytoplasm did not occur and these neuroepithelial cells lost their columnar morphology.
Keywords: methionine, protein methylation, neural tube closure, cytoskeletal proteins
During neurulation cells of the developing nervous system become tissue specific as neural folds rise from the flat neural plate and turn inward at their tips to form the neural tube. Recent studies have focused on factors involved with inductive interactions such as the protein of the sonic hedgehog gene that has appeared to be involved with both the formation of the floor plate and the motor neurons (1). Interest also has focused on protein factors containing DNA binding transcription motifs that have been implicated in the direct regulation of gene expression (2). For example, the Pax-3 gene encodes three DNA binding transcription factors and has appeared unique as its expression along the dorsal length of the neural folds as well as along the floor and alar plates was coincident with neural tube closure (3). Mutations in the murine homologue of Pax-3 (Splotch) resulted in exencephaly and spina bifida (3, 4), while mutations in the human homologue of this same gene (Waardenburg syndrome type I) (5) also have been associated with neural tube defects (6). Nevertheless, linkage analyses have failed to demonstrate that PAX-3 is a major gene in the general occurrence of neural tube defects (7). Recent knockout studies in mice for the AP-2 transcription factor also have resulted in exencephaly along with a wide variety of other defects (8, 9).
Several different mutations have been reported for the human reductase gene involved in the transfer of methyl groups from 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate in the pathway leading to the formation of methionine from the methylation of homocysteine (10, 11). These mutations caused elevated levels of circulating homocysteine, reduced levels of methionine, and an increased frequency of neural tube defects (12). As dietary supplements of folic acid have been reported to reduce the occurrence and recurrence of neural tube defects by as much as 50 to 75% (13, 14) these reductase mutations could provide a basis for an increased requirement for this vitamin. However, because estimates have suggested that only 13% of neural tube defects could be attributed to the reductase mutations (12), other causes should be considered.
When whole rat embryos undergoing neurulation were cultured on a medium of blood sera from epileptic subjects, neural tubes failed to close with some samples unless cultures were supplemented with methionine (15). These sera were low in free methionine when compared with other sera that supported normal neural tube closure. When embryos were cultured on bovine sera their neural tubes also failed to close unless methionine was added to the culture, and, again, this source of sera contained either low or undetectable amounts of free methionine (16). In these studies with bovine sera the methyl group from methionine appeared essential as only choline–chloride, also a methyl group donor, provided partial replacement of the amino acid. In addition, several observations suggested that protein rather than DNA methylation was the relevant reaction, and, indeed, hypomethylation of neural tube proteins was observed (17). This included reductions in levels of dimethylarginine and 3-methylhistidine in neural tube proteins of embryos cultured on bovine sera without added methionine in comparison with either neural tube proteins of methionine-supplemented embryos or heart proteins from methionine-deficient embryos. In the present study these observations were extended to identify specific neural tube proteins that became methylated before neural tube closure and to determine the effects of methionine availability on the intracellular distribution of these proteins in the developing neuroepithelium.
MATERIALS AND METHODS
Embryo Culture.
Embryos were isolated from CD strain rats (Charles River Breeding Laboratories) at 9.5 days of gestation, Reichert’s membranes were removed, and only those embryos at the midhead-fold stage were selected for experiments (18, 19). Two embryos were cultured in a single culture tube containing 1.33 ml of medium consisting of bovine sera that had been prepared from blood immediately centrifuged following collection from mature nonpregnant Holstein cows maintained at the University of Connecticut. Serum was heat-inactivated, filtered for sterility, and used at a concentration of 90% (vol/vol) in combination with 10% water that contained supplements of glucose (3 mg/ml), antibiotics, and, where indicated, l-methionine (25 μg/ml) (Sigma). Culture tubes were rotated at 30 rpm, maintained at 37.5°C, and gassed with mixtures of CO2, O2, and N2 as described (17).
After 40 hr of culture, embryos from two culture tubes were combined (four embryos) into a single culture tube, incubated in Tyrode’s solution containing puromycin (0.075 mg/ml) (Sigma) for 1 hr, and then 20 μCi (1 Ci = 37 GBq) of either [35S]methionine (specific activity 1037 Ci/mmol) or [methyl-14C]methionine (specific activity 51 mCi/mmol) (DuPont) for a final 5 hr of incubation. Extraembryonic membranes were removed and embryos were rinsed in PBS before further analyses.
It should be noted that responses of the embryos to culture on bovine serum were highly consistent. In the presence of methionine, bovine sera supported neural tube closure, normal optic cup development for this stage, and a “C”-shaped body curvature. In the absence of methionine, neural tubes remained open from the trunk region to the cranium and anophthalmia (absence of eyes); also, incomplete body curvature was observed.
Inhibition of Protein Synthesis.
To determine the maximum inhibition of protein synthesis possible without allowing breakdown of embryo tissues, embryos were cultured for 40 hr on bovine serum, exposed to increasing concentrations of puromycin (0.025–0.1 mg/ml) (Sigma) in Tyrode’s solution for 1 hr, and then incubated with [35S]methionine for another 5 hr. Four embryos were homogenized in 25 μl Tyrode’s solution, 5 μl aliquots were used for protein determination (Bradford protein assay kit; Bio-Rad), and 10 μl aliquots in combination with 500 μl bovine serum albumin (carrier) (2 mg/ml) (Sigma) were incubated with 500 μl of 10% trichloroacetic acid (Sigma) at 4°C overnight. Precipitates were rinsed twice with ethanol and air dried on glass microfiber filter disks (Whatman GF/C 24 mm), and 5 ml of Ecolite(+) scintillation fluid (ICN Biomedicals) was added before determining radioactivity (Beckman LS1801 scintillation counter).
At 0.025 mg/ml puromycin incorporation of [35S]methionine was 78% (average of three determinations) of that observed without puromycin, and at 0.1 mg/ml embryo tissue disintegration was apparent. A concentration of 0.075 mg/ml was selected for experiments as incorporation was only 33% (average of three determinations) of that observed without puromycin, and embryos remained intact during exposure to the drug.
[Methyl-14C]methionine Labeling and Two-Dimensional Gel Electrophoresis.
After 40 hr of culture on bovine serum, four embryos were combined into a single culture tube containing Tyrode’s solution and puromycin (0.075 mg/ml) for 1 hr and then [methyl-14C]methionine (20 μCi) was added for another 5 hr of incubation. Embryos were rinsed and homogenized in 25 μl of Tyrode’s solution, 5 μl aliquots were used for determining trichloroacetic acid-precipitable radioactivity, and aliquots were further solubilized in 9.4 M urea and treated with 1 μl/50 μl sample of an equal mixture of DNase I and RNase A. For two-dimensional gel electrophoresis, equal amounts of total radioactivity (range, 18,000–25,000 cpm) from puromycin treated and nontreated embryos were loaded onto isoelectric focusing tubes (protein did not exceed 100 μg per tube) (20). In the second dimension, 10% SDS/PAGE wase used (21). Gels were examined after silver staining (22), equilibrated overnight in glycerol-acetic acid solution, soaked in Enlightning (DuPont) for 20 min, dried, and exposed to Kodak X-Omat X-ray film for periods up to 8 weeks at −70°C (20).
Two-Dimensional Gel Western Immunoblot.
Following two-dimensional electrophoresis, proteins were transferred to Immobilon-P transfer membrane (Millipore) (23). The membrane was incubated overnight in 1% powdered milk in PBS, incubated with antisera for either neurofilament L (Sigma), αβ-tubulin (ICN Biomedicals), or β/γ-actin (gift from J. Lessard, University of Cincinnati) diluted 1:20 in PBS–Tween (pH 7.5) shaken for 2 hr and rinsed in PBS–Tween. The membrane was incubated with a 1:100 dilution of peroxidase-linked goat anti-rat IgG (Sigma) in PBS–Tween for another 2 hr, rinsed, and air-dried before being developed with 0.2 mmol/liter N-chloro-1-naphthol in 25 mmol/liter citrate buffer.
Thin Layer Chromatography.
Embryo protein homogenates were first hydrolyzed 6 M HCl overnight at 110°C (24). The amino acid standards, dimethylarginine, 3-methylhistidine, mono-plus trimethyllysine, and methionine (Sigma), were mixed with the radioactive embryo protein hydrolysates, chromatographed (developing solution pyridine/acetone/NH4OH/H2O at 10:6:1:4), and then visualized by staining with 1% ninhydrin (Alltech Associates) in acetone. Areas (1.0 cm2) of the cellulose plate (catalog no. 891010, Selecto Scientific, Norcross, GA) that completely contained the stained amino acid spots were cut out from the glass backing and incubated with 5 ml Ecolite(+) scintillation fluid (ICN Biomedicals) for 30 min before the determination of radioactivity.
Indirect Immunofluorescence.
Embryos were fixed in Carnoy’s solution, dehydrated in alcohol, and embedded in paraffin. Sections cut at 5 μm were deparaffinized in xylol and rehydrated in an alcohol series. The sections were incubated with antisera for β/γ-actin, αβ-tubulin, or neurofilament L diluted at 1:80 in PBS–Triton X-100 (0.05%) (pH 7.4) for 3 hr, rinsed twice in PBS–Triton X-100, and incubated for 3 hr with fluorescein isothiocyanate-linked rat anti-rabbit antibodies (Sigma) diluted 1:40 before mounting with coverslips. A Zeiss Axioplan microscope with epiilumination was used to examine and photograph sections.
RESULTS
Protein Identification.
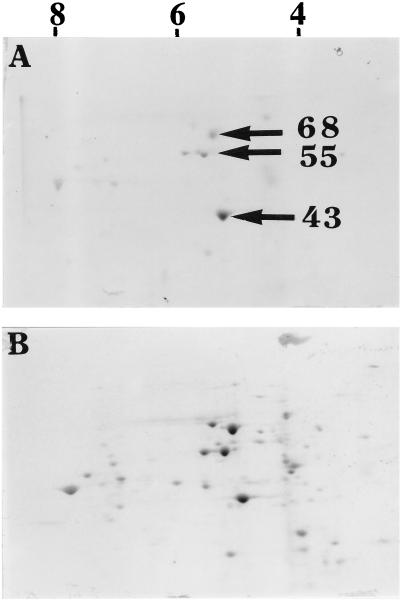
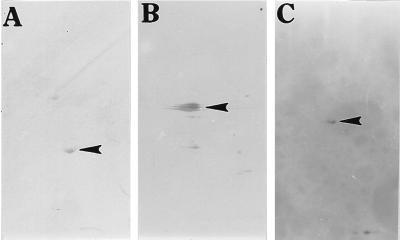
To identify proteins that became methylated during neurulation, whole head-fold stage rat embryos were first cultured for 40 hr on methionine-deficient bovine serum to deplete methionine pools, transferred for 1 hr to Tyrode’s solution containing puromycin (0.075 mg/ml) to limit protein synthesis (protein synthesis was only 33% of that observed without the inhibitor), and then incubated for a final 5 hr with puromycin and [methyl-14C]methionine. When silver stained two-dimensional polyacrylamide gels of proteins from puromycin-treated and untreated whole embryos were compared, differences in the distribution patterns of protein spots were not apparent. However, when these same two-dimensional gels (the gels were run at equal counts per minute) were exposed to x-ray film for periods up to 8 weeks and compared, puromycin treatment was found to greatly reduce the number of different [methyl-14C]methionine-labeled protein spots. In each of four separate experiments, three spots (one appearing as two closely adjacent spots) were considered the darkest and were selected for further analyses (Fig. 1). Based on their estimated molecular masses (43, 55, and 68 kDa), isoelectric points (5.1, 5.8, and 5.6, respectively), analyses by the Expert Protein Analysis System (Swiss-Prot) data base, presence in the developing nervous system (25), and known posttranslational modifications (26), they were believed to represent actin, αβ-tubulin, and neurofilament L, respectively. Using antibodies specific for each of these three proteins with two-dimensional gel Western immunoblots, the assumed identities of these methylated proteins were confirmed (Fig. 2) as the antibody spots could be precisely aligned with those spots developed from the x-ray film (Fig. 1A).
Figure 1.
Two-dimensional gel electrophoresis of [methyl-14C]methionine-labeled embryo proteins. After 40 hr of culture rat embryos were preincubated with or without puromycin for 1 hr, then labeled with [methyl-14C]methionine for 5 hr in the continued presence (A) and absence (B) of puromycin. Dry two-dimensional gels were exposed to x-ray film at −70°C for 8 weeks. For the first dimension the range of isoelectric points are indicated by numbers at the top, and for the second dimension specific molecular weights in kDa are indicated by arrows.
Figure 2.
Two-dimensional gel electrophoresis Western immunoblots using antibodies to actin, αβ-tubulin, and neurofilament L. Rat embryos were cultured for 48 hr, their proteins separated by two-dimensional PAGE, Western blotted, and detected with antibodies specific for (A) actin (43 kDa, pI 5.1), (B) αβ-tubulin (55 kDa, pI 5.6), and (C) neurofilament L (68 kDa, pI 5.8). Spots are indicated by arrowheads.
Protein Methylation.
As the silver-stained two-dimensional gels of the three proteins identified by [methyl-14C]methionine labeling also appeared the darkest, two approaches were used to distinguish protein methylation from synthesis. First, comparisons were made between embryo proteins labeled with [methyl-14C]methionine and [35S]methionine using puromycin and the experimental design previously described (40 hr culture, 1 hr puromycin, and 5 hr terminal labeling). The two-dimensional gels failed to clearly distinguish incorporation of the radioactivity of [methyl-14C]methionine from that of [35S]methionine as the same three spots were predominant with both labeled precursors. This suggested that the three proteins were not only methylated but also represented proteins that were being synthesized most actively at this stage of development.
In the second approach, the same experimental design again was used with [methyl-14C]methionine but embryo proteins were now hydrolyzed (6 M HCl at 110°C in sealed vials overnight) and then the amino acids mixed with added standards were separated by thin layer chromatography and stained with 1% ninhydrin. The stained spots on the cellulose plate were isolated and subjected to radioactive counting, and the amounts of radioactivity corresponding to mono-plus trimethyllysine, 3-methylhistidine, dimethylarginine, and methionine were found to exceed background radioactivity (44 cpm) by 4-, 4-, 2-, and 8-fold, respectively.
Protein Distribution.
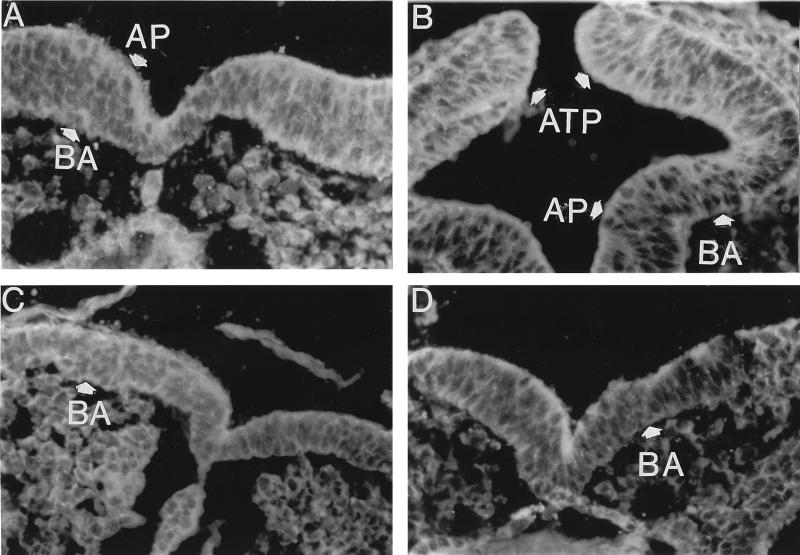
After 18 hr of embryo culture on bovine sera supplemented with methionine, the binding of fluorescently labeled actin antibodies was most apparent on the apical (lumen side) and to a lesser extent in the basal region of the cytoplasm of cells of the cranial neural ectoderm (Fig. 3A). Considerably less actin antibody binding was apparent in the adjoining epidermal ectoderm, the heart, cells surrounding blood vessels, and the mesenchymal cells (not seen at this magnification). After 24 hr of culture actin antibody binding was more clearly noted in the apical and now in the basal regions of the neural ectoderm cells as well (Fig. 3B). At the tissue level, after 24 hr the most intense fluorescence was observed in the cells of the apposing tips of the neural folds. It should be noted that although not clearly apparent in the photographs, the cells at the apposing tips of the neural folds appeared elongated and wedge-shaped. In contrast to these methionine-supplemented embryos, without methionine striking reductions in actin antibody binding particularly in the basal regions of these cells occurred after 18 hr and such binding was essentially absent after 24 hr of culture (Fig. 3 C and D). Additionally, concentrations of actin antibody binding in the cells of the potential apposing tips observed with methionine was not observed in the absence of methionine supplements. The cells as well as their nuclei were not elongated as with methionine supplements, but rather most cells and their nuclei appeared round in shape. It should also be noted that without methionine supplementation the aligned columnar nature of the neural ectoderm was lost in some regions.
Figure 3.
Cellular distribution of actin antibody binding in the cranial neural ectoderm of neurulating rat embryos after 18 and 24 hr of culture. Indirect immunofluorescence sections with actin antibody of embryos cultured on bovine sera that were either methionine-supplemented (A and B) or unsupplemented (C and D), and after either 18 hr (A and C) or 24 hr (B and D) of culture. AP, apical (lumen side) side of the neural ectoderm cells; BA, basal (side distal to the lumen) side of the neural ectoderm cells; ATP, apposing tips of the neural folds. (×500.)
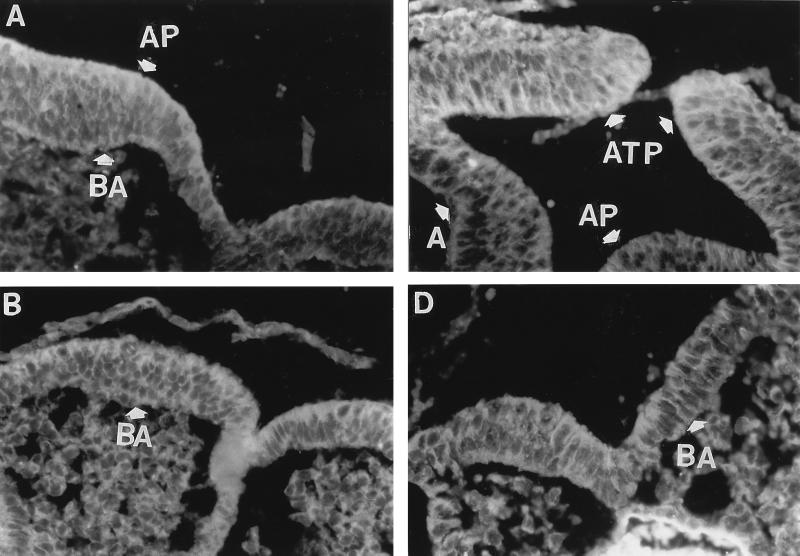
In the presence of methionine, the αβ-tubulin antibody binding to the apical and basal cytoplasm of the cranial neural ectoderm cells appeared similar to that of actin antibody binding. However, at 18 hr the αβ-tubulin antibody binding could be observed to be more uniformly distributed throughout the cytoplasm than was observed with the actin antibody (Fig. 4A). After 24 hr of culture, again like the actin antibody, αβ-tubulin antibody binding was particularly evident in the cells at the apposing tips of the neural folds (Fig. 4B). In contrast to methionine-supplemented embryos, without this supplement the αβ-tubulin antibody binding in the apical cytoplasm of neural ectoderm cells was less apparent and in the basal cytoplasm it was essentially absent (Fig. 4 C and D).
Figure 4.
Cellular distribution of αβ-tubulin antibody binding in the cranial neural ectoderm of neurulating rat embryos after 18 and 24 hr of culture. Indirect immunofluorescence of sections with αβ-tubulin antibody of embryos cultured on bovine sera that were either methionine-supplemented (A and B) or unsupplemented (C and D) at 18 hr (A and C) or 24 hr (B and D) of culture. AP, apical (lumen side) side of neural ectoderm cells and basal (BA) (side distal to the lumen) side of neural ectoderm cells; ATP, apposing tips of the neural folds. (×500.)
As with others studies (27–29), the binding of commercially available antibodies to the neurofilament L protein used in this study could not be detected after 18 or 24 hr of culture, and this was not improved by treating the sections with reagents such as proteases and detergents.
DISCUSSION
In the present study, by culturing whole rat embryos on methionine-deficient bovine sera and limiting protein synthesis with puromycin, it was possible to identify three proteins labeled with [methyl-14C]methionine during neurulation: actin, αβ-tubulin, and neurofilament L. As [35S]methionine labeling also indicated that these three proteins were among the most abundantly synthesized at this developmental stage, amino acid analyses of embryo proteins were used to demonstrate that embryo proteins could be methylated at this early stage of development. However, insufficient amounts of embryo protein precluded attempts to directly analyze the individual proteins. Additionally, it should be noted that the methods used probably would not be expected to detect methylation of minor proteins.
Although extensively studied, except for bacterial chemotaxis in which protein methylation through an S-adenosyl methionine-dependent pathway causes directional flagellar rotation in response to chemical stimuli, a functional role for protein methylation has been difficult to establish (30). Indeed, replacement of the highly conserved histidine that becomes methylated at position 73 of actin (31) or the lysine that becomes methylated at position 394 of tubulin (32) did not alter a variety of functions for these proteins including their incorporation into the cellular cytoskeleton or assembly in vitro. Nevertheless, a possible role for methylation in processes such as the interactions of these proteins with other molecules could not be excluded in these studies.
Although no attempt was made here to establish a role for methylation in the function of actin, αβ-tubulin, or neurofilament L, the importance of cytoskeletal proteins in cell morphology and particularly in neural tube closure has been implicated for some time. For example, when mouse embryos in culture were exposed to a variety of agents that disrupted cytoskeletal polymerization, the neural ectoderm cells did not assume their characteristic wedge shape and neural tubes failed to close (33). In addition, the binding of antibodies to actin in the apical cytoplasm of these cells was previously observed and suggested to play a key role in cellular morphogenesis as well as in the process of neural tube closure (25). In the present study the apical location of actin was confirmed and, in addition, αβ-tubulin antibody binding also was localized in this region. However, most striking was the failure to observe the basal cytoplasm localization of actin and αβ-tubulin in the absence of methionine supplements. This lack of basal antibody binding was associated with rounding of the cells as well as their nuclei, and, again, failure of the neural tubes to close. The absence of protein localization was possibly due to a lack of adequate interaction between the various proteins that form focal adhesions. One such candidate could be the guanosine triphosphate binding protein, rho, which has been observed to induce a rapid reorganization of actin filaments from a punctate form of distribution to a more diffuse network of stress fibers (34). Furthermore, it was interesting to note that this ras-related protein has been found to require an S-adenosyl methionine dependent carboxyl-methylation itself to localize properly at the inner surfaces of cell membranes (35).
In addition to providing new insights into the mechanisms of neurulation, the present study may also provide a basis for the variety of factors that have been reported to be involved with neural tube closure including nutrient deficiency, drug exposure, and faulty genes. For example, the previously reported reductions in human neural tube closure defects attributable to dietary folate supplements (13, 14) may be indicative of methionine deficiency as the methyl group of folic acid in combination with homocysteine forms methionine. Alternatively, methionine deficiency also could result from blockage of nutrient transport as has been demonstrated for antibodies to the extracellular matrix protein laminin (36). On the other hand, the anticonvulsant drug, valproic acid, that has been known to cause neural tube defects in humans (37) did not appear to limit methionine uptake in rats (38), but still the embryo toxicity could be overcome by additional methionine. This suggested that valproic acid might have reduced the efficiency of an enzyme or enzymes involved with the methylation cycle. Indeed, when rat embryos were exposed in culture to valproic acid the cytoskeletal proteins actin and αβ-tubulin failed to localize in the basal cytoplasm of the neural ectoderm cells similar to that observed here with methionine deficiency (unpublished observation). Regarding genes, to date only the Axd mouse mutant susceptible to neural tube defects has been reported to respond to methionine supplements with reductions in the occurrence of neural tube defects (39). Methionine supplements would also be expected to circumvent the incidence of neural tube defects caused by mutations in the 5,10-methylenetetrahydrofolate reductase enzyme. Hopefully, the present study will lead to further investigation and insights into the interactions of the many steps in protein methylation with environmental and genetic factors during neurulation.
Acknowledgments
We thank Dr. J. Lessard (Developmental Biology Division, University of Cincinnati) for the gift of actin antibodies as well as Mary Bruno and Carol Norris for assistance with two-dimensional electrophoresis gels. This work was supported by funds from the National Institute on Environmental Health Sciences (Grant ESO4312 to N.W.K.), the Research Foundation of the University of Connecticut, and the Storrs Agricultural Experiment Station. This is scientific contribution number 1691 from the Storrs Agricultural Experiment Station.
References
- 1.Roelink H, Porter J A, Chiang C, Tanabe Y, Chang D T, Beachy P A, Jessell T M. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 2.Gruss P, Walther C. Cell. 1992;69:719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- 3.Goulding M D, Chalepakis G, Deutsch U, Erselius J R, Gruss P. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein D J, Vekemans M, Gros P. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin C T, Hoth C F, Amos J A, da-Silva E O, Milunsky A. Nature (London) 1992;355:637–638. doi: 10.1038/355637a0. [DOI] [PubMed] [Google Scholar]
- 6.Tassabehji M, Read A P, Newton V E, Harris R, Balling R, Gruss P, Strachan T. Nature (London) 1992;355:635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- 7.Chatkupt S, Hol F A, Shugart Y Y, Geurds M P A, Stenroos E S, Koenigsberger M R, Hamel B C J, Johnson W G, Mariman E C M. J Med Genet. 1995;32:200–204. doi: 10.1136/jmg.32.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell P J. Nature (London) 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon A P, Flavell R A, Williams T. Nature (London) 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- 10.Goyette P, Frosst P, Rosenblatt D S, Rozen R. Am J Hum Genet. 1995;56:1052–1059. [PMC free article] [PubMed] [Google Scholar]
- 11.van der Put N M J, Steegers-Theunissen R P M, Frosst P, Trijbels F J M, Eskes T K A B, van den Heuvel L P, Mariman E C M, Heyer M-den, Rozen R, Blom H J. Lancet. 1995;346:1070–1071. doi: 10.1016/s0140-6736(95)91743-8. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead A S, Gallagher P, Mills J L, Kirke P N, Burke H, Molloy A M, Weir D G, Shields D C, Scott J M. Q J Med. 1995;88:763–766. [PubMed] [Google Scholar]
- 13.MRC-Vitamin Study Research Group. Lancet. 1991;338:131–154. [PubMed] [Google Scholar]
- 14.Czeizel A D, Dudas I. New Engl J Med. 1992;26:1832–1837. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 15.Chatot C L, Klein N W, Clapper M L, Resor S R, Singer W D, Russman B S, Holmes G L, Mattson R H, Cramer J A. Epilepsia. 1984;25:204–216. doi: 10.1111/j.1528-1157.1984.tb04177.x. [DOI] [PubMed] [Google Scholar]
- 16.Coelho C N D, Weber J A, Klein N W, Daniels W H, Hoagland T A. J Nutr. 1989;119:1716–1725. doi: 10.1093/jn/119.11.1716. [DOI] [PubMed] [Google Scholar]
- 17.Coelho C N D, Klein N W. Teratology. 1990;42:437–451. doi: 10.1002/tera.1420420412. [DOI] [PubMed] [Google Scholar]
- 18.New D A T, Coppola P T, Cockroft D L. J Reprod Fertil. 1976;48:219–222. doi: 10.1530/jrf.0.0480219. [DOI] [PubMed] [Google Scholar]
- 19.Klein N W, Vogler M A, Chatot C L, Pierro L J. Teratology. 1980;21:199–208. doi: 10.1002/tera.1420210211. [DOI] [PubMed] [Google Scholar]
- 20.O’Farrell P H. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Norris C E, diIorio P J, Schultz R J, Hightower L E. Mol Biol Evol. 1995;12:1048–1062. doi: 10.1093/oxfordjournals.molbev.a040280. [DOI] [PubMed] [Google Scholar]
- 23.Towbin H, Staechlin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Lazarides E, O’Connor C M, Clarke S. J Biol Chem. 1982;257:8356–8362. [PubMed] [Google Scholar]
- 25.Sadler T W, Lessard J L, Greenburg D, Coughlin P. Science. 1982;215:172–174. doi: 10.1126/science.7031898. [DOI] [PubMed] [Google Scholar]
- 26.Szasz J, Burns R, Sternlicht H. J Biol Chem. 1982;257:3697–3704. [PubMed] [Google Scholar]
- 27.Bennett G S, DiLullo C. Dev Biol. 1985;107:94–106. doi: 10.1016/0012-1606(85)90379-3. [DOI] [PubMed] [Google Scholar]
- 28.Cochard P, Paulin D. J Neurosci. 1984;4:2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carden M J, Trojanowski J Q, Schlaepfer W W, Lee V M-Y. J Neurosci. 1987;7:3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stock J. In: Protein Methylation. Paik W K, Kim S, editors. Boca Raton, FL: CRC; 1990. pp. 346–367. [Google Scholar]
- 31.Solomon L R, Rubenstein P A. J Biol Chem. 1987;262:11382–11388. [PubMed] [Google Scholar]
- 32.Szasz J, Yaffe M B, Sternlicht H. Biophys J. 1993;64:792–802. doi: 10.1016/S0006-3495(93)81440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Shea, K. S. (1986) Scanning Electron Microsc. 1195–1213. [PubMed]
- 34.Ridley A J, Hall A. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 35.Philips M R, Pillinger M H, Staud R, Volker C, Rosenfeld M G, Weissmann G, Stock J B. Science. 1993;259:977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- 36.Chambers B J, Klein N W, Nosel P G, Khairallah L H, Romanow J S. J Nutr. 1995;125:1487–1599. doi: 10.1093/jn/125.6.1587. [DOI] [PubMed] [Google Scholar]
- 37.Robert E, Guibaud P. Lancet. 1982;ii:937. doi: 10.1016/s0140-6736(82)90908-4. [DOI] [PubMed] [Google Scholar]
- 38.Nosel P G, Klein N W. Teratology. 1992;46:499–507. doi: 10.1002/tera.1420460514. [DOI] [PubMed] [Google Scholar]
- 39.Essien F B, Wannberg S L. J Nutr. 1993;123:27–34. doi: 10.1093/jn/123.1.27. [DOI] [PubMed] [Google Scholar]