Abstract
Aim
To determine if there is a relationship between the amount of increase in IOP following dilatation with a cycloplegic agent and the future course of glaucoma.
Method
A retrospective chart review of 100 eyes from 55 subjects with open‐angle glaucoma who had had IOP measured before and after pharmacological pupillary dilatation was performed to establish the rate of progression of glaucoma, based on serial evaluation of the visual fields using the glaucoma staging system 2 (GSS 2), and optic discs using the disc damage likelihood scale (DDLS). Progressive visual field loss was defined as an increase of two or more stages with the GSS 2 and progressive deterioration of the disc was defined as an increase of two or more stages with the DDLS. Mean follow‐up time was 7.2 years.
Results
A total of 26 eyes showed glaucomatous progression. The likelihood of progression of glaucoma was related to the amount of IOP increase after pharmacological pupillary dilatation. For every 1 mmHg increase in IOP, the odds of progression increased 24% (p = 0.008). The likelihood of progression of glaucoma, however, was not related to the baseline IOP, which was 20.63 mmHg (SD = 4.59 mmHg) in those showing deterioration of disc or field and 19.72 mmHg (SD = 5.32 mmHg) in those not worsening according to our definition.
Conclusion
In patients with open‐angle glaucoma, the amount of increase in IOP caused by pharmacological pupillary dilatation is related to the likelihood of future progression of glaucoma.
The disability caused by glaucoma is due to the disease's progressive damage to the optic nerve. A goal of treatment is to prevent such progression.1 Knowing which patients are likely to get worse is therefore of great importance, and consequently many prognostic factors have been considered.2,3,4 Here, we evaluate the possibility that the degree of elevation of intraocular pressure (IOP) following dilatation of the pupil might be a useful prognostic indicator, the value of which is increased by its simplicity and low cost.
Materials and methods
We performed a retrospective chart review of 100 eyes from 55 subjects aged 21 years or more with open‐angle glaucoma who had been seen in the Glaucoma Service Department of Wills Eye Institute (Philadelphia, Pennsylvania, USA). Patients with angle‐closure glaucoma, anatomically narrow angle, any condition or disease that affects pupillary dilatation, and any condition other than glaucoma that decreases the visual field or visual acuity or causes optic nerve cupping were excluded from the study. Also excluded from the study were patients with less than 5 years of follow‐up. Data collected from each patient's chart included age, gender, type of glaucoma, visual acuity on the first and last visit, Goldmann applanation IOP before and after dilatation on the first visit, Goldmann applanation IOP on the last visit, and the follow‐up period. The criteria for progression of glaucoma was based on serial evaluation of the visual fields (using the glaucoma staging system 2 (GSS 2)5 that has a range of 0–5) and the optic discs (using the disc damage likelihood scale (DDLS),6 a classification scheme that stages glaucomatous optic nerve damage based upon the narrowest radial width of neural rim and vertical disc diameter, and which has a range of 1–10). GSS 2 and DDLS scores were collected for the first and last visit of each patient. GSS 2 scores were assigned retrospectively based on the mean defect and corrected pattern standard deviation of the patients' visual fields, taken at the first and last visit, as described earlier.5 DDLS scores used in this study had already been recorded in the chart of each patient for the first and last visit by the treating physician. Progressive visual field loss was defined as an increase of two or more stages with the GSS 2 and progressive deterioration of the disc was defined as an increase of two or more stages with the DDLS.
Logistic regression analysis was used to assess the relationship between the incidence of progression and the change in IOP after pupillary dilatation. Standard errors of parameter estimates were adjusted to account for using multiple eyes from the same subjects using generalised estimating equation (GEE) methods. Student's t‐test was used to compare means.
Results
The patients included 30 women and 25 men. A total of 21 patients were less than 70 years old and 34 patients were 70 years old or more (mean ± SD: 73.1±11.7 years; range: 46–98 years). The mean follow‐up time was 7.2 years (range: 5–15 years).
The average DDLS score for all eyes was 4.0±1.8. For those who progressed, the average was 3.8±1.8, and for those who did not progress 4.0±1.9. A total of 78 eyes (78%) had a DDLS score of five or less, and 22 eyes (22%) had a DDLS score of six or more.
The average GSS 2 score for all eyes was 2.0±1.2. For those who progressed, the average was 1.5±1.0, and for those who did not progress 2.0±1.2.
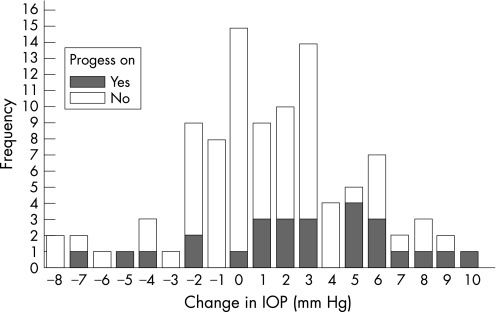
In total, 74 eyes (74%) in 38 patients did not show progression and 26 eyes (26%) in 17 patients showed progression according to our criteria (fig 1). In all, 24 eyes (24%) in 15 patients showed progression with the DDLS, and 4 eyes (4%) in four patients showed progression with the GSS 2. Two eyes (2%) in two patients showed progression in DDLS and GSS 2.
Figure 1 Bar graph of the frequency of progression and non‐progression in relation to change of intraocular pressure after dilatation.
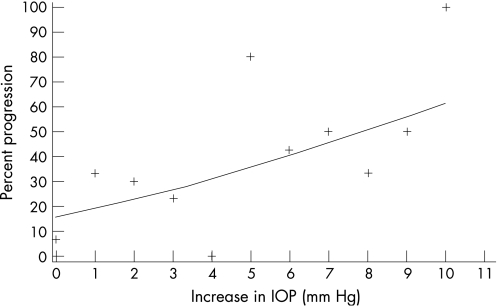
The change in IOP after dilatation ranged from −8 to +10 mmHg. The likelihood of progression of glaucoma was related to the amount of IOP increase after pharmacological pupillary dilatation (fig 2). For every 1 mmHg increase in IOP, the odds of progression increased 24% (p = 0.008). This relationship was independent of the baseline IOP, which was 20.6±4.6 mmHg in those showing glaucomatous progression of the disc or field, and 19.7±5.3 mmHg in those not worsening, according to our definition. There was no significant relationship between age and progression or change in IOP postdilatation.
Figure 2 Scatter plot of progression of glaucoma against increase in intraocular pressure after dilatation.
Discussion
Mydriatic drugs can cause a significant rise in IOP in patients with open‐angle glaucoma.7 Kristensen showed that 48% of eyes with open‐angle glaucoma had an IOP rise of 8 mmHg or more when dilated with 10% phenylephrine.8 Harris demonstrated that approximately 23% of patients with primary open‐angle glaucoma had a significant rise in IOP following administration of cycloplegics.9 Lee found IOP elevations of up to 27 mmHg in selected patients with open‐angle glaucoma following pupillary dilatation.10 Studies have suggested that the rise in IOP reaches its maximum between 45 and 120 min after cyclopentolate drops are administered, and lasts for 4–6 h if left untreated.9,11 The exact mechanism by which this increase in pressure takes place is not known, though it might result from pigment liberation into the anterior chamber and subsequent obstruction of the trabecular meshwork.8 Alternatively, it might result from decreased pull on the trabecular meshwork due to ciliary muscle paralysis, leading to a drop in aqueous outflow.11
The stage of glaucoma in a person is presently based on consideration of the amount of visual field loss and/or optic nerve damage. When the field or the disc shows increased damage the glaucoma is said to be “progressed.” Progression has been related to the level12 and fluctuation13,14,15 of IOP. It is reasonable to assume that eyes with greater rise in IOP postdilatation have higher IOP to start with. However, this was not the case in our study. Therefore, it is not likely that the worse clinical course in the cases in the present study is due to having a higher IOP in those who progressed than those who were stable. Change in IOP with pupillary dilatation is a type of IOP fluctuation. It is reasonable to assume that the eyes with changes in IOP following dilatation had larger than usual fluctuations of IOP in other times.
In our study, we found that likelihood of progression of glaucoma was related to the amount of IOP rise after pharmacological pupillary dilatation (p = 0.008). This progression was independent of baseline IOP and age, in contradiction to other studies.12,16,17 As control of IOP is largely a function of the nature of the trabecular drainage system,18 our results suggest that the amount of IOP increase secondary to dilatation can be used as a surrogate measure for some factors related to progression, possibly trabecular function.
There was a large discrepancy in the number of eyes showing progression according to the different methods used to determine this, the DDLS or the GSS 2. There are a number of possible explanations. The DDLS is a system of quantifying the amount of optic nerve change from none (stage 1) to end stage (stage 10). The GSS 2 is a method of staging the amount of visual field loss, from no loss (stage 0) to end stage loss (stage 5). Because each DDLS stage is “smaller” than a GSS 2 stage, less glaucomatous change is needed to define an eye as having progressed when using DDLS than when using GSS 2. Furthermore, 78 eyes were scored as 5 or lower on the DDLS scale, indicating early glaucoma, while only 22 eyes were scored as 6 or more. Changes in the optic disc can be seen before the occurrence of perimetric abnormalities,19 so it is to be expected that in some cases progressive change in the disc will be seen in the absence of field change.20 Progression was considered present only if one or both of these systems, DDLS and GSS 2, changed by more than one scale unit. This change was considered sufficient evidence for progression because, for DDLS, intra‐observer variability has been established as less than two scale units.6 With the GSS2 there should be “no” intra‐observer variability, because the system is based on using numbers on a field chart. However, there is variability in the results of field tests when they are repeated. These variabilities might be small and well within the range of normal fluctuation but yet large enough to result in the first field being graded in a different stage from a repeat field. Therefore, in order to be sure that progression has occurred, that is, that the second field is actually worse than the first, we required a change in the GSS2 of two units. The sensitivity of this system is thus much lower than the sensitivity of the DDLS.
A shortcoming of this study is its retrospective design, which can lead to bias. However, because the data were based on notes written at the time of the patients' earlier visits, and were simply collected and analysed retrospectively, we do not believe that this is a problem. DDLS scores were already noted on the patients' charts and GSS 2 scores were assigned objectively using information on the Humphrey visual fields that had already been performed.
Another possible shortcoming in this study is that most of the patients had two eyes enrolled. Because two eyes of a single patient are not fully independent, it is possible that the effect of IOP change on the odds of progression could be overestimated in this study. However, a statistical analysis method (GEE) was employed to lessen this problem.
In conclusion we found that in patients with open‐angle glaucoma, the amount of increase in IOP caused by pharmacological pupillary dilatation is related to the likelihood of future progression of glaucoma.
Acknowledgement
Statistical help was provided by Dr Ben Leiby of Jefferson medical college, Philadelphia, Pennsylvania, USA.
Abbreviations
DDLS - disc damage likelihood scale
GEE - generalised estimating equation
GSS - glaucoma staging system
IOP - intraocular pressure
Footnotes
Funding: This study was supported by the Glaucoma Foundation to Prevent Blindness.
Competing interests: None.
References
- 1.Jampel H D. Glaucoma patients' assessment of their visual function and quality of life. Tr Am Ophth Soc 200199301–317. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown K E, Congdon N G. Corneal structure and biomechanics: impact on the diagnosis and management of glaucoma. Curr Opin Ophthalmol 200617338–343. [DOI] [PubMed] [Google Scholar]
- 3.Jonas J B, Budde W M, Stroux A.et al Iris colour, optic disc dimensions, degree and progression of glaucomatous optic nerve damage. Clin Exp Ophthalmol 200634654–660. [DOI] [PubMed] [Google Scholar]
- 4.Gordon M O, Beiser J A, Brandt J D.et al The ocular hypertension treatment study. Arch Ophthalmol 2002120714–720. [DOI] [PubMed] [Google Scholar]
- 5.Brusini P, Filacorda S. Enhanced glaucoma staging system (GSS 2) for classifying functional damage in glaucoma. J Glaucoma 20061540–46. [DOI] [PubMed] [Google Scholar]
- 6.Bayer A, Harasynowycz P, Henderer J D.et al Validity of a new scale for estimating glaucomatous damage: Correlation with visual field damage. Am J Ophthalmol 2002133758–763. [DOI] [PubMed] [Google Scholar]
- 7.Shaw B R, Lewis R A. Intraocular pressure elevation after pupillary dilation in open angle glaucoma. Arch Ophthalmol 19861041185–1188. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen P. Pigment liberation test in open‐angle glaucoma. Acta Ophthalmol 196846586–589. [DOI] [PubMed] [Google Scholar]
- 9.Harris L S. Cycloplegic‐induced intraocular pressure elevations a study of normal and open‐angle glaucomatous eyes. Arch Ophthalmol 196879242–246. [DOI] [PubMed] [Google Scholar]
- 10.Lee P F. The influence of epinephrine and phenylephrine on intraocular pressure. Am Med Assoc Arch Ophthalmol 195860863–867. [DOI] [PubMed] [Google Scholar]
- 11.Joos K M, Kay M D, Pillunat L E.et al Effect of acute intraocular pressure changes on short posterior ciliary artery haemodynamics. Br J Ophthalmol 19998333–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leske M C, Heijl A, Hussein M.et al Factors for glaucoma progression and the effect of treatment. Arch Ophthalmol 200312148–56. [DOI] [PubMed] [Google Scholar]
- 13.Wilensky J T. The role of diurnal pressure measurements in the management of open angle glaucoma. Curr Opin Ophthalmol 20041590–92. [DOI] [PubMed] [Google Scholar]
- 14.Saccà S C, Rolando M, Marletta A.et al Fluctuations of intraocular pressure during the day in open‐angle glaucoma, normal‐tension glaucoma and normal subjects. Ophthalmologica 1998212115–119. [DOI] [PubMed] [Google Scholar]
- 15.Phelps C D, Phelps G K. Measurement of intraocular pressure: A study of its reproducibility. Graefes Arch Clin Exp Ophthalmol 197619839–43. [DOI] [PubMed] [Google Scholar]
- 16.Lichter P R, Musch D C, Gillespie B W.et al Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology 20011081943–1953. [DOI] [PubMed] [Google Scholar]
- 17.Stewart W C, Kolker A E, Sharpe E D.et al Factors associated with long‐term progression or stability in primary open‐angle glaucoma. Am J Ophthalmol 2000130274–279. [DOI] [PubMed] [Google Scholar]
- 18.Ethier C R, Kamm R D, Palaszewski B A.et al Calculations of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci 1986271741–1750. [PubMed] [Google Scholar]
- 19.Danesh‐Meyer H V, Gaskin B J, Jayusundera T.et al Comparison of disc damage likelihood scale, cup to disc ratio, and Heidelberg retina tomography in the diagnosis of glaucoma. Br J Ophthalmol 200690437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read R, Spaeth G L. Practical clinical appraisal of the optic disc in glaucoma: The natural history of cup progression and some specific disc field correlations. Trans Am Acad Ophthalmol Otolaryngol 197478255–274. [PubMed] [Google Scholar]