Abstract
Background/Aims
The repeatability of an automatic system for estimation of endothelial cell density (ECD) from microscopy images in donor corneas was assessed.
Methods
A computer program for the automatic ECD estimation from frequency analysis was previously presented. An evaluation of its repeatability was performed on a data set containing 200 corneas by assessing the accuracy and precision of automatic versus manual values. For each cornea, 2–21 images (1536 total) at 100× for automatic ECD and one image at 200× for manual ECD were available.
Results
Accuracy of automatic ECDs was −45 (SD 99) cells/mm2 (−2% (4%)). Precision of repeated automatic ECDs on the same cornea was 62 (30) cells/mm2 (3% (1%)). The algorithm was also capable of identifying all non‐processable images.
Conclusion
The proposed automatic technique proved to be reliable for its routine use in a typical eye bank setting like the one considered here.
At eye banks, endothelial cell density (ECD) is estimated from microscopy images of donor corneas using a tedious, highly subjective and error‐prone manual procedure.1 Several prototypes aiming at the automatic extraction of cell contours and analysis of corneal endothelium have been proposed.2,3,4,5 However, as images are often blurred and noisy, an accurate, fully automatic recognition of cell contours is hard to achieve and none of these systems is known to be in routine clinical use at a significant number of institutions.
We recently introduced a fully automated system to estimate ECD,6 based on a spatial frequency analysis and not requiring the difficult step of cell contour detection. We present the results of a study to assess its repeatability—that is, the capability of providing a reproducible ECD when analysing multiple images from the same cornea.
Material and methods
The procedure routinely used at Cornea Bank Berlin Charitè was followed. Corneas were put in hypotonic BSS for microscopy visualisation of the endothelial cells. Images were acquired before organ culture (33%) or after de‐swelling (67%) in organ culture medium (modified Eagle's medium with 2% fetal calf serum) containing 6% Dextran 500, in order to have a low amount of folds of the Descemet membrane and a large area of cells in focus. Only corneas with visible endothelial cells after osmotic stimulation were used.
Images were acquired from 200 donor corneas using an inverted phase‐contrast microscope (CK 40, Olympus, Japan) at 100× magnification and an analogue camera (SSC‐DC50AP, Sony, Japan), and digitised as 768×576 pixels grey‐level images. The framed area was 1256×940 μm (area size 1.180 mm2) and was located in the central zone of the cornea, to avoid bias in the cell density evaluation.7 Several digital images were acquired, with the possibility of partial overlapping between them; the mean (standard deviation) number of replicates for each cornea was 8 (SD 3) (range 2–21).
Endothelial cell density estimation
Endothelial cell density was estimated by an automatic procedure based on a spatial frequency analysis using the 2D Discrete Fourier Transform (DFT).6 DFT extracts from an image information regarding the various spatial frequencies it contains—that is, about the repetitive patterns present in the image. The magnitude of these various frequency components can be represented as an image, which for corneal endothelium images exhibits a specific pattern—a bright circular band around the origin. This may be interpreted as the image having the frequencies inside this band as dominant frequencies.
As this band is symmetric around the origin, we can reduce the extraction of frequency information from two dimensions to one dimension, deriving a frequency signal that represents the DFT magnitude as a function of the spatial frequency. Two peaks are clearly identifiable in this signal: the position of the second one was found to provide the frequency of the repetitive cell pattern and thus ECD.6
The algorithm also allows assessment of when image quality is not adequate to derive a correct ECD. In these cases, the frequency signal always contains more than two peaks and this feature was used to detect non‐processable images.
Repeatability evaluation
The procedure above was applied to estimate ECD in all images from the 200 donor corneas. No manual pre‐selection was applied. Accuracy of these ECDs was evaluated by comparison with reference values, and precision, as indicator of repeatability, was determined as the standard deviation (SD) of densities from replicate images of the same cornea.
We chose to use as reference values the manual counts performed by eye bank experts. To this end, one digital image was also acquired from the central area of each cornea at 200× magnification with a framed area of 628×470 μm (area size 0.295 mm2). The higher magnification allowed a better manual identification of cells. By observing these images on a computer monitor and selecting a rectangular region of interest (ROI) occupying approximately 50% of the acquired image, visible cells were manually counted by two experienced investigators, for a total of 400 estimations. Images with good cell visibility were used and only cells with clearly visible borders and entirely within the ROI, or touching one or both of the lower and left border, were counted.
For each cornea, the mean automatic ECD from all 100× images and the mean manual ECD from the two experts' counts were derived. These values were used to compute accuracy of the automatic method.
Results
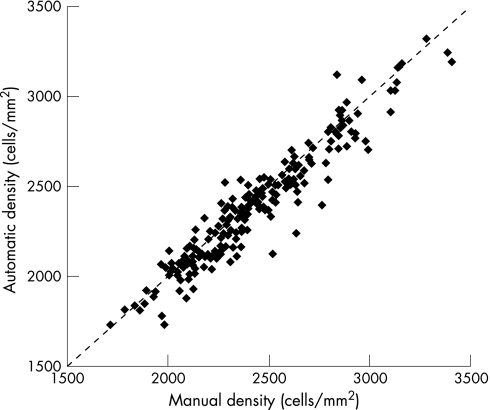
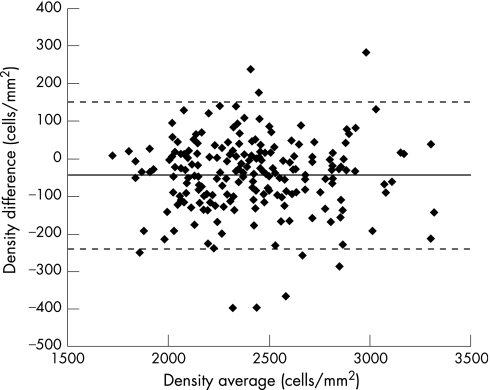
The software was able to screen out 14% of images as non‐processable. None of the accepted images provided an unacceptably inaccurate ECD—that is, with a difference from manual greater than 20%. Accuracy, expressed for each cornea as the difference between automatic and manual mean ECDs, was on average −45 (SD 99) cells/mm2 (−2% (4%)) (table 1). Figure 1 reports a scatter‐plot of automatic versus manual ECDs for all corneas and compares it with the identity line. Figure 2 represents a plot of differences against averages of the same densities:8 the distribution of differences is independent of density values with 95% limits of agreement of −239÷150 cells/mm2.
Table 1 Summary results on accuracy of automatic ECD estimation.
| Manual | Automatic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp 1 | Exp 2 | Mean | Total, n | Processed, n | % Processed | Mean | Diff | % Diff | |
| Mean | 2430 | 2444 | 2437 | 8 | 7 | 86 | 2392 | −45 | −2 |
| SD | 335 | 332 | 327 | 3 | 3 | 18 | 327 | 99 | 4 |
| Min | 1695 | 1741 | 1718 | 2 | 2 | 13 | 1726 | −397 | −16 |
| Max | 3480 | 3363 | 3406 | 21 | 16 | 100 | 3319 | 282 | 10 |
For the 200× image of each cornea (Manual), manual densities were obtained by two experts (Exp 1 and Exp 2) and then their mean (Mean) was derived. For all 100× images of each cornea (Automatic), total number of images for each cornea (Total, n) and number and percentage of processed images (Processed, n; % Processed) are reported; automatic densities were obtained and then their mean (Mean), difference (Diff), and per cent difference (% Diff) from the manual mean were computed. For all quantities, mean, standard deviation, minimum and maximum values are reported over the 200 corneas. All quantities are in cells/mm2, except when “n” or “%” is indicated.
Figure 1 Scatter‐plot of average manual versus average automatic endothelium cell density estimates. The dotted line indicates the line of identity.
Figure 2 Scatter‐plot of difference versus average for each pair of (average) manual and (average) automatic densities. The dashed line shows the average difference, the dotted lines show the 95% limits of agreement.8
For the repeatability analysis (table 2), the precision was expressed as the standard deviation of ECDs obtained in replicate images of the same cornea: on average 68 (59) cells/mm2 (3% (2%)) for manual counts and 62 (30) cells/mm2 (3% (1%)) for automatic densities. The repeatability coefficient9 was derived and its value was 171 cells/mm2 for automatic and 187 cells/mm2 for manual ECDs. It is worth pointing out that repeating the automatic procedure many times on the same image would be pointless, as the results would be exactly the same.
Table 2 Summary results on precision (repeatability) of automatic estimation of endothelial cell density.
| Manual | Automatic | |||
|---|---|---|---|---|
| SD (%) | Range (%) | SD (%) | Range (%) | |
| Mean | 68 (3) | 95 (4) | 62 (3) | 163 (7) |
| SD | 59 (2) | 83 (3) | 30 (1) | 86 (4) |
| Min | 0 (0) | 0 (0) | 7 (0) | 13 (1) |
| Max | 270 (11) | 382 (16) | 156 (7) | 455 (21) |
For the 200× image of each cornea (Manual), standard deviation (SD) and range were derived from the manual densities obtained by two experts. For all 100× images of each cornea (Automatic), automatic densities were obtained and then their standard deviation and range were computed. Per cent values for all quantities are also reported. For all quantities, average, SD, minimum and maximum values are reported over the 200 corneas. All quantities are in cells/mm2, except when “%” is indicated.
Automatic ECD estimation on an Intel Pentium 4 computer required approximately one second per image.
Discussion and conclusion
The satisfactory results on accuracy of the first evaluation6 were confirmed in this 15‐fold larger image data set (1536 total images). The main goal of the present study was however the assessment of the repeatability of automatic density estimation, as it is important to obtain density values very close to one another when multiple images are acquired in different areas of the central cornea, where the density is approximately the same.
Even if in the same cornea we are not comparing identical situations (multiple experts on same image vs same algorithm on multiple images), SDs and repeatability coefficients9 from multiple densities can still provide the basis to assess and compare repeatability. The results obtained by the automatic procedure are very good and even slightly better than those from our manual procedure. They are definitely better than the ones reported in other studies involving multiple manual counts.10 It should also be noted that the repeatability in multiple automatic densities is also affected by the physiological variation of ECD in different regions within the central cornea,11 which is not present for the manual densities, where the two experts analysed the same image.
Whereas the manual counts were performed on an ROI half the size of a 200× image, the automatic estimations were both derived from whole 100× images, with an eight times larger ROI, and also averaged from many such images. Multiple automatic estimations are thus much less affected by local bias than a single manual estimation. It should be mentioned that in many eye banks the routine procedure entails a manual estimation performed on a much smaller area than used here, typically 1/100th of mm2, and thus the local bias of its density estimation is further exacerbated. Conversely, images from peripheral areas, which are largely out of focus due to the curvature of the cornea, are automatically rejected by the program, preventing the user from obtaining a falsely high cell count.
The results of this study were obtained on images collected at only one eye bank. Although they are likely to hold for any other eye bank adopting the same protocol for cornea preservation and analysis, they need to be confirmed in other eye bank settings.
Acknowledgments
The authors wish to thank Mrs C Jaeckel, Cornea Bank Berlin Charitè, for collaboration in the image evaluation.
Abbreviations
DFT - Discrete Fourier Transform
ECD - endothelial cell density
ROI - region of interest
Footnotes
Competing interests: A Ruggeri is a paid consultant for Nidek Technologies, Italy.
References
- 1.Bourne W M. Examination and photography of donor corneal endothelium. Arch Ophthalmol 1976941799–1800. [DOI] [PubMed] [Google Scholar]
- 2.Lester J M, MacFarland J L, Bursell S E.et al Automated morphometric analysis of corneal endothelial cells. Invest Ophthalmol Vis Sci 198120407–410. [PubMed] [Google Scholar]
- 3.Siertsema J V, Landesz M, Van den Brom H.et al Automated video image morphometry of corneal endothelium. Doc Ophthalmol 19938535–44. [DOI] [PubMed] [Google Scholar]
- 4.Corkidi G, Marquez J, Usisima R.et al Automated in vivo and online morphometry of human endothelium. Med Biol Eng Comput 199331421–426. [DOI] [PubMed] [Google Scholar]
- 5.Gain P, Thuret G, Gavet Y.et al Automated tri‐image analyser of stored corneal endothelium. Br J Ophthalmol 200286801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri A, Grisan E, Jaroszewski J. A new system for the automatic estimation of endothelial cell density in donor corneas. Br J Ophthalmol 200589306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann J, Holley G P, Lee S.et al Increased endothelial cell density in the paracentral and peripheral regions of the human cornea. Am J Ophthalmol 2003135584–590. [DOI] [PubMed] [Google Scholar]
- 8.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 9.Bland J M, Altman D G. Measuring agreement in method comparison studies, Stat Methods Med Res 19998135–160. [DOI] [PubMed] [Google Scholar]
- 10.Thuret G, Acquart S, Manissolle C.et al Urgent need for normalization of corneal graft quality controls in French eye banks. Transplantation 2004781299–1302. [DOI] [PubMed] [Google Scholar]
- 11.Waring G O, Bourne W M, Edelhauser H F. The corneal endothelium: normal and pathologic structure and function. Ophthalmology 198289531–590. [PubMed] [Google Scholar]