Abstract
Aims
To study the visual prognosis and ocular characteristics of eyes with polypoidal choroidal vasculopathy (PCV) that appear to have classic choroidal neovascularisation (CNV) on fluorescein angiography (FA).
Methods
The authors reviewed retrospectively 38 eyes with PCV that appear to have classic CNV on FA. Lesions were examined with indocyanine green angiography, FA and optical coherence tomography (OCT).
Results
In all cases OCT showed subretinal material with moderate reflectivity that corresponded in location to classic CNV. At the final visit, the subretinal material resolved completely in 14 eyes (36.8%, resolved group), but resolved only incompletely in 24 eyes (63.2%, persisted group) after photodynamic therapy (PDT). Mean (standard deviation) visual acuity in the resolved group (0.35 (0.41) in log MAR) was significantly better than that in the persisted group (0.84 (0.24)) at the final visit (p<0.001). The subretinal material seen before treatment was more frequently seen in subfovea in the persisted group (87.5% vs 42.9%, p = 0.007). Also, this material was located adjacent to polypoidal lesions more often in the resolved group (92.9% vs 58.3%, p = 0.030).
Conclusions
Eyes with PCV sometimes show classic CNV with subretinal material apparent on OCT, and PCV is thus attributed to type 2 CNV or to pure fibrinous tissue without CNV. Visual prognosis in eyes with type 2 CNV is poor, and although it is difficult to discriminate type 2 CNV from pure fibrin deposition before treatment, type 2 CNV is seen more often in the subfovea and is typically separate from the polypoidal lesions.
Polypoidal choroidal vasculopathy (PCV) is characterised by a branching vascular network that terminates in polypoidal lesions.1,2,3,4,5,6,7,8 To date, the pathogenesis of PCV is not fully understood9 but has been reported to originate from an abnormality of the inner choroidal vessels.1,2,3,10 As most components of the PCV are located beneath the retinal pigment epithelium (RPE), they appear typically as occult choroidal neovascularisation (CNV) on fluorescein angiography (FA).11,12,13
However, PCV sometimes accompanies type 2 CNV.1,6,10,14,15 Previous histological examinations of surgical specimens from eyes with PCV showed fibrovascular tissue within Bruch's membrane with secondary CNV in the subretinal space (type 2 CNV).16,17,18,19,20 Type 2 CNV in eyes with PCV appears in most cases as greyish subretinal material, most of which is shown to be classic CNV on FA.15,21 Optical coherence tomography (OCT) shows type 2 CNV to consist primarily of subretinal material with moderate reflectivity.22,23 In most cases, the visual prognosis of eyes with subfoveal type 2 CNV is poor. Even after successful treatment with photodynamic therapy (PDT), most type 2 CNV does not disappear completely, but rather remains as subfoveal fibrotic tissue with resultant poor vision.23
In contrast, in some eyes with PCV that appears as classic CNV on FA, the subretinal material with moderate reflectivity detected by OCT disappears completely after PDT. Recently, Otsuji et al15 reported that some PCV eyes with an appearance of classic CNV, in fact show that the subretinal material just over the polypoidal lesions consists solely of fibrinous tissue, with no CNV. Leakage from active polypoidal lesions can result in fibrin deposition in the subretinal space, which may simulate classic CNV on FA. This deposited fibrin usually disappears after PDT, and visual prognosis is thus quite good.
Therefore, when eyes with PCV show classic CNV on FA, it would be of great help to determine whether they do in fact have type 2 CNV, in order to better predict the visual prognosis. However, before treatment it is often difficult to discriminate type 2 CNV from pure fibrin deposition, even using detailed examination by OCT. We believe, however, that the reaction to PDT is quite different; after treatment, most type 2 CNV does not disappear completely (persisted group), while purely fibrinous tissue often resolves completely (resolved group). In this study, we investigated the visual prognosis and other ocular characteristics of eyes with PCV and apparently classic CNV, based on the reaction to PDT of the subretinal material seen on OCT images. Based on our findings, we report differences between the two groups and the key factors that help to differentiate them before treatment.
Patients and methods
For this interventional case study, we reviewed 38 eyes of 38 patients with PCV appearing as classic CNV who visited the Macular Service at Kyoto University Hospital from August 2004 to January 2006. All patients had undergone comprehensive ophthalmological examination, including best‐corrected visual acuity, intraocular pressure, indirect ophthalmoscopy, slit‐lamp biomicroscopy with contact lens, and OCT examination. Two types of OCT (Stratus OCT3000; Carl Zeiss, Dublin, CA, USA and OCT ophthalmoscope C7; Nidek, Gamagori, Japan) were used. After fundus photographs were taken, FA and indocyanine green angiography (IA) were performed on each patient using a confocal laser scanning system (HRA‐2; Heidelberg Engineering, Dossenheim, Germany).
Institutional review board/ethics committee approval was not required for this retrospective study.
The diagnosis of PCV was based on IA, which typically shows a branching vascular network that terminates in polypoidal swelling. The polypoidal lesion may be a single polyp or a cluster of multiple polyps. In most cases, the reddish‐orange nodules that were seen under ophthalmoscopic examination corresponded to polypoidal lesions seen by IA. Eyes with other macular abnormalities (that is, age‐related macular degeneration (AMD), pathologic myopia, idiopathic CNV, presumed ocular histoplasmosis, angioid streaks, and other secondary CNV) were excluded from the current study.
Patients were offered PDT if they had sustained visual loss due to exudative changes secondary to subfoveal and juxtafoveal PCV. The risks and benefits of the treatment were explained to all patients and informed consent was obtained before treatment. PDT was performed using a 689 nm diode laser unit (Visulas PDT system 690S; Carl Zeiss) after an injection of verteporfin (Visudyne; Novartis, Bülach, Switzerland), according to PDT guidelines for AMD. The laser irradiation spot was decided adding 1000 μm to the lesion size based on FA and IA. All polypoidal lesions, leaking vascular network, and classic CNV detected with FA or IA were included. The entire area of large haemorrhagic or serous detachment of the RPE was not necessarily included in the laser irradiation spot if no polypoidal lesion or CNV could be detected with IA.
Every three months, all patients underwent comprehensive ophthalmological examination, including best‐corrected visual acuity, indirect ophthalmoscopy, slit‐lamp biomicroscopy with contact lens, OCT examination, and FA and IA. When residual or recurrent fluorescein leakage was observed in the treated eye, at scheduled three month follow‐up visits, retreatment was performed.
All values are presented as mean (standard deviation). Statistical analysis was performed using χ2 tests and the unpaired t test. Differences were considered statistically significant when the p values were less than 0.05. Change in best‐corrected visual acuity was considered to be significant when the change in visual acuity, in logMAR fashion, was equal to or greater than 0.3.
Results
In the current study, we examined 38 eyes of 38 patients (29 men and 9 women) with PCV appearing as classic CNV. The patients ranged in age from 53 to 90 years (70.6 (8.7) years) and all were Japanese. The visual acuity of eyes with PCV ranged from 20/500 to 20/30 (median, 20/100). In all eyes, IA showed polypoidal lesions, most of which were connected to a branching vascular network. On FA, all of 38 eyes revealed lesions that showed classic CNV on FA (figs 1–4). Most of these showed an area of choroidal hyperfluorescence that could be discerned in the early phase of FA, and occasionally the early phase showed the capillary network of the CNV. In the later phases of FA, progressive pooling of fluorescein leakage occurred, and usually obscured the boundaries of the CNV.
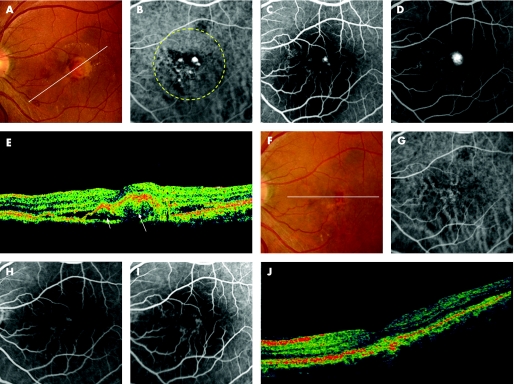
Figure 1 A 50‐year‐old man (patient 5) was referred to our clinic with a two‐month history of metamorphopsia in the left eye (OS). At the initial visit, his visual acuity was 20/15 OS. Funduscopic examination of the left eye revealed a reddish‐orange nodule with overlying greyish material in the posterior pole. At three months after the initial visit, the reddish‐orange nodule with overlying greyish material and subretinal haemorrhage has increased in size and his visual acuity decreased to 20/130 (A). Indocyanine green angiography (IA) reveals a branching vascular network terminating in polypoidal lesions. (B). Fluorescein angiography (FA) shows juxtafoveal classic choroidal neovascularisation (CNV) corresponding to the largest polypoidal lesion seen on IA (C, early phase; D, late phase). A sectional image with optical coherence tomography (OCT) along the white line shows a juxtafoveal polypoidal lesion (short arrow) and adjacent subretinal material with moderate reflectivity (long arrow) (E). He was treated with photodynamic therapy (PDT) in the left eye. The yellow dotted line indicates the laser irradiation spot. Fundus photograph at six months after PDT shows neither the reddish‐orange nodule nor the overlying grey material (F). IA shows no polypoidal lesions (G). FA shows no classic CNV (H, early phase; I, late phase). Neither the subretinal material nor the polypoidal lesion are detected on sectional imaging with OCT along the white line (J). His visual acuity was 20/200 OS.
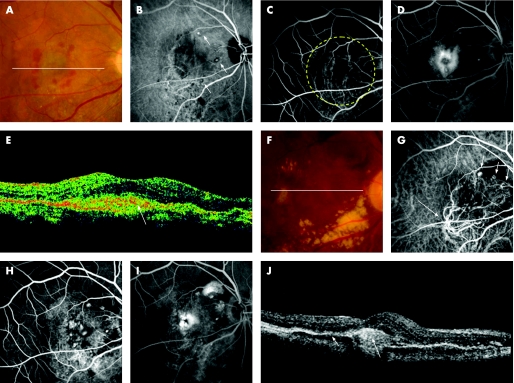
Figure 2 A 63‐year‐old woman (patient 8) was referred to our clinic with a six‐month history of metamorphopsia and decreased visual acuity in the left eye (OS). At the initial visit, her visual acuity was 20/30 OS and funduscopic examination revealed a reddish‐orange nodule with overlying greyish material in the posterior pole (A). Indocyanine green angiography (IA) reveals a branching vascular network terminating in polypoidal lesions, which are juxtafoveal in location (B). Fluorescein angiography (FA) shows classic juxtafoveal choroidal neovascularisation (CNV) corresponding to the polypoidal lesion seen on IA (C, early phase; D, late phase). A sectional image with optical coherence tomography (OCT) along the white line shows a polypoidal lesion (arrow) and adjacent subretinal material with moderate reflectivity (long arrow) (E). She was treated with photodynamic therapy (PDT) in the left eye. The yellow dotted line indicates the laser irradiation spot. A fundus photograph at three months after PDT shows neither reddish‐orange nodules nor overlying greyish material (F). IA shows no polypoidal lesions (G). FA shows no classic CNV (H, early phase; I, late phase). OCT image along the white line shows reduced polypoidal lesion (arrow) (J). Her visual acuity was 20/13 OS.
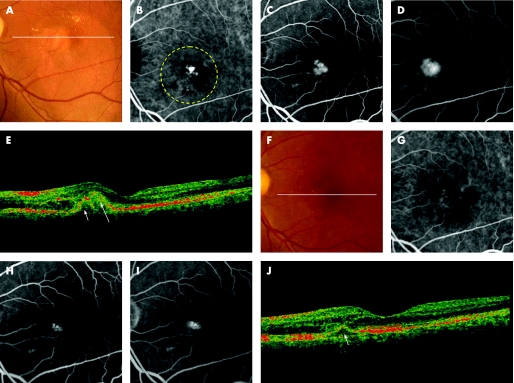
Figure 3 A 72‐year‐old man (patient 24) was referred to our clinic with a one‐month history of decreased visual acuity in the left eye (OS). At the initial visit, his visual acuity was 20/100 OS. Funduscopic examination of the left eye reveals a reddish‐orange nodule (arrow) and subretinal greyish material with subretinal haemorrhage in the posterior pole (A). Indocyanine green angiography (IA) reveals a branching vascular network terminating in an extrafoveal polypoidal lesion (B). Fluorescein angiography (FA) shows subretinal classic choroidal neovascularisation (CNV), which is separate from the polypoidal lesion (C, early phase; D, late phase). A sectional image with optical coherence tomography (OCT) along the white line shows an elevation of retinal pigment epithelium (short arrow) and subretinal material with moderate reflectivity (long arrow) (E). He was treated with photodynamic therapy (PDT) to the left eye. The yellow dotted line indicates the laser irradiation spot at the first treatment. Three treatments with PDT were performed to the persisted classic CNV. Fundus photograph at nine months after the first PDT shows subretinal greyish material (F). IA shows no polypoidal lesions (G). FA shows persisted subfoveal classic CNV (H, early phase; I, late phase). An OCT image along the white line shows persisted moderate reflectivity subfoveally (arrow) (J). His visual acuity was 20/100 OS; this patient was reported in a previous article.29
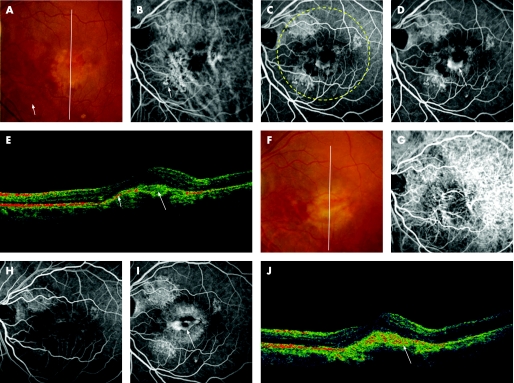
Figure 4 A 67‐year‐old man (patient 19) was referred to our clinic with a six‐month history of metamorphopsia in the right eye (OD). At the initial visit, funduscopic examination of the right eye revealed only serous retinal detachment, and visual acuity was 20/25 OD. At one year after the initial visit, the exudative change had increased in the right eye, and his visual acuity decreased to 20/200 OD. Funduscopic examination of the right eye reveals subretinal haemorrhage with subretinal greyish material in the posterior pole (A). Indocyanine green angiography (IA) reveals a branching vascular network terminating in three extrafoveal polypoidal lesions (arrows) (B). Fluorescein angiography (FA) shows subfoveal classic choroidal neovascularisation (CNV), which is separate from the polypoidal lesions seen on IA (C, early phase; D, late phase). A sectional image with optical coherence tomography (OCT) along the white line shows subfoveal subretinal material with moderate reflectivity (long arrow) (E). He was treated with photodynamic therapy (PDT) in the right eye. The yellow dotted line indicates the laser irradiation spot at the first treatment. Two treatments with PDT and an intravitreal injection of triamcinolone acetonide were performed for the persisted classic CNV. A fundus photograph at 18 months after the first PDT shows subfoveal greyish material and extensive subretinal hard exudate (F). IA shows a branching vascular network terminating in three polypoidal lesions (arrows) and the root of the network (long arrow) (G). FA shows residual classic CNV subfoveally (H, early phase; I, late phase). OCT image along the white line shows residual moderate reflectivity (long arrow) and a slight elevation of the retinal pigment epithelium (arrow), both of which correspond to a branching vascular network (J). His visual acuity was 20/130 OD.
On OCT, most of the reddish‐orange nodules seen on the fundus were detected as round protrusions of RPE. When the IA image was overlaid on the OCT image, the polypoidal lesions were often detected as a protrusion of the RPE. In all eyes that showed classic CVN on FA, OCT examination showed the subretinal material with moderate reflectivity, or occasionally with high reflectivity, located between the high reflective line of RPE and neurosensory retina, corresponding to classic CNV. In the current study, all of these 38 eyes were treated with PDT and the follow‐up after PDT was 14.0 (4.6) months (range 5–22 months). At the final visit, OCT revealed complete resolution of the subretinal material in 14 (36.8%) of the 38 eyes (resolved group; figs 1 and 2); in 24 eyes (63.2%), however, the subretinal material had not disappeared completely (persisted group; figs 3 and 4).
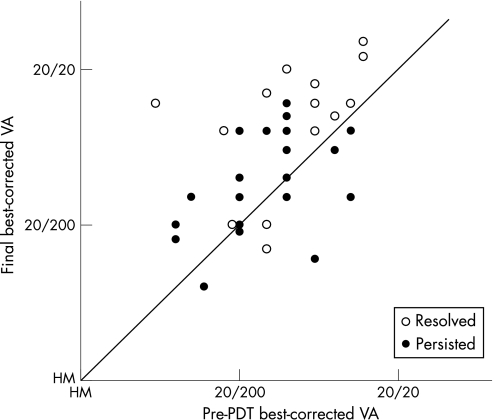
Tables 1 and 2 show the characteristics of both groups. The percentages of men and the average ages in the resolved and persisted groups were 78.6% and 75.0% (p = 1.000), and 70.4 (11.0) and 70.8 (7.3) years (p = 0.760), respectively. Mean visual acuity before PDT was 0.68 (0.37) in the resolved group and 0.81 (0.33) in the persisted group; no significant difference was seen in visual acuity before PDT between the two groups (p = 0.279). Figure 5 shows the change in visual acuity in the two groups. At the final visit, visual acuity in the resolved group (0.35 (0.41)) was significantly better than that in the persisted group (0.84 (0.24)) (p<0.001). While only two (14.3%) of 14 eyes showed decreased visual acuity in the resolved group, six (25.0%) of 24 eyes in the persisted group showed decreased visual acuity after PDT. In addition, significant visual recovery was observed at the final visit in 8 (57.1%) of 14 eyes in the resolved group and in four (16.7%) of 24 eyes in the persisted group (p = 0.026). Also at the final visit, although no eye in the resolved group showed classic CNV on FA, 12 (50.0%) of 24 eyes in the persisted group showed classic CNV.
Table 1 Characteristics of patients in resolved group.
| Patient | Age | Sex | Location of classic CNV | Relation of classic CNV to polypoidal lesion | LS (μm) | PDT treatments, n | BCVA at baseline | BCVA at final visit | Follow‐up duration (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | F | Juxtafovea | Adjacent | 2900 | 1 | 20/250 | 20/50 | 22 |
| 2 | 73 | M | Juxtafovea | Adjacent | 750 | 1 | 20/70 | 20/25 | 21 |
| 3 | 70 | F | Subfovea | Adjacent | 2650 | 1 | 20/130 | 20/30 | 21 |
| 4 | 68 | M | Subfovea | Adjacent | 3000 | 3 | 20/70 | 20/50 | 18 |
| 5 | 50 | M | Juxtafovea | Adjacent | 2450 | 1 | 20/130 | 20/200 | 17 |
| 6 | 68 | M | Juxtafovea | Adjacent | 1330 | 2 | 20/30 | 20/15 | 15 |
| 7 | 56 | M | Subfovea | Adjacent | 3060 | 1 | 20/100 | 20/20 | 14 |
| 8 | 63 | F | Juxtafovea | Adjacent | 1900 | 1 | 20/30 | 20/13 | 14 |
| 9 | 80 | M | Subfovea | Adjacent | 2090 | 1 | 20/130 | 20/300 | 13 |
| 10 | 68 | M | Juxtafovea | Adjacent | 1960 | 2 | 20/50 | 20/40 | 13 |
| 11 | 80 | M | Subfovea | Adjacent | 2100 | 2 | 20/220 | 20/200 | 12 |
| 12 | 80 | M | Juxtafovea | Adjacent | 3600 | 1 | 20/40 | 20/35 | 10 |
| 13 | 90 | M | Juxtafovea | Separated | 2750 | 1 | 20/70 | 20/35 | 8 |
| 14 | 59 | M | Subfovea | Adjacent | 2300 | 1 | 20/500 | 20/35 | 5 |
CNV, choroidal neovascularisation; LS, lesion size; PDT, photodynamic therapy; BCVA, best‐corrected visual acuity.
Adjacent, adjacent to polypoidal lesions; separated, separated from polypoidal lesions.
Table 2 Characteristics of patients in the persisted group.
| Patient | Age | Sex | Location of classic CNV | Relation of classic CNV to polypoidal lesion | LS (μm) | PDT treatments, n | BCVA at baseline | BCVA at final visit | Follow‐up duration (months) |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 81 | M | Subfovea | Adjacent | 2900 | 2 | 20/100 | 20/130 | 21 |
| 16 | 73 | M | Subfovea | Separated | 770 | 4 | 20/200 | 20/200 | 21 |
| 17 | 72 | M | Juxtafovea | Adjacent | 2400 | 4 | 20/40 | 20/130 | 20 |
| 18 | 53 | F | Subfovea | Adjacent | 3100 | 3 | 20/100 | 20/130 | 20 |
| 19 | 67 | M | Subfovea | Separated | 2200 | 2 | 20/200 | 20/130 | 19 |
| 20 | 73 | M | Subfovea | Adjacent | 2880 | 2 | 20/200 | 20/100 | 18 |
| 21 | 66 | M | Subfovea | Adjacent | 2850 | 2 | 20/70 | 20/300 | 18 |
| 22 | 74 | M | Subfovea | Separated | 4200 | 2 | 20/400 | 20/200 | 15 |
| 23 | 73 | M | Subfovea | Adjacent | 2850 | 3 | 20/100 | 20/100 | 14 |
| 24 | 72 | M | Subfovea | Separated | 3300 | 3 | 20/100 | 20/100 | 14 |
| 25 | 73 | M | Subfovea | Separated | 4400 | 1 | 20/200 | 20/200 | 14 |
| 26 | 79 | M | Juxtafovea | Adjacent | 1710 | 1 | 20/100 | 20/70 | 13 |
| 27 | 73 | M | Subfovea | Adjacent | 5400 | 1 | 20/300 | 20/500 | 13 |
| 28 | 68 | M | Subfovea | Separated | 4720 | 1 | 20/40 | 20/50 | 13 |
| 29 | 74 | F | Subfovea | Adjacent | 2000 | 2 | 20/200 | 20/250 | 12 |
| 30 | 72 | M | Subfovea | Separated | 3550 | 3 | 20/50 | 20/70 | 12 |
| 31 | 75 | M | Subfovea | Adjacent | 2100 | 1 | 20/200 | 20/200 | 12 |
| 32 | 80 | M | Subfovea | Adjacent | 3600 | 1 | 20/500 | 20/250 | 11 |
| 33 | 74 | F | Subfovea | Adjacent | 2310 | 2 | 20/130 | 20/100 | 10 |
| 34 | 63 | F | Subfovea | Adjacent | 3560 | 2 | 20/500 | 20/200 | 10 |
| 35 | 72 | M | Subfovea | Separated | 3700 | 1 | 20/100 | 20/100 | 9 |
| 36 | 55 | F | Juxtafovea | Separated | 4500 | 2 | 20/40 | 20/130 | 8 |
| 37 | 58 | F | Subfovea | Separated | 1500 | 1 | 20/100 | 20/70 | 7 |
| 38 | 79 | M | Subfovea | Adjacent | 4500 | 1 | 20/100 | 20/100 | 6 |
CNV, choroidal neovascularisation; LS, lesion size; PDT, photodynamic therapy; BCVA, best‐corrected visual acuity.
Adjacent, adjacent to polypoidal lesions; separated, separated from polypoidal lesions.
Figure 5 Correlation between best‐corrected visual acuity at baseline and at the final visit in eyes with polypoidal choroidal vasculopathy with an appearance of classic choriodal neovascularisation (CNV). Open circles indicate visual acuity of eyes in which classic CNV resolved after photodynamic therapy (PDT). Closed circles indicate visual acuity of eyes in which the classic CNV persisted after PDT. VA, visual acuity; HM, hand motion.
Location of the subretinal material that corresponded to classic CNV on FA was examined with FA, IA, and OCT (table 3). Before treatment, the subretinal material was seen most often in the subfoveal area (27 (71.1%) of 38 eyes). The subretinal material that persisted after PDT were more frequently seen in subfovea preoperatively; the subretinal materials were located subfovealy in six (42.9%) of 14 eyes in the resolved group, and in 21 (87.5%) of 24 eyes in the persisted group (p = 0.007). The subretinal material was situated in many cases adjacent to the polypoidal lesions seen on IA (27 (71.1%) of 38 eyes). The subretinal material seen adjacent to the polypoidal lesions before treatment often disappeared after PDT; the subretinal material adjacent to the polypoidal lesions was seen in 13 of 14 eyes (92.9%) in the resolved group but in only 14 of 24 eyes (58.3%) in the persisted group (p = 0.030).
Table 3 Comparisons of resolved group and persisted group.
| Resolved group n = 14 | Persisted group n = 24 | p Value | |
|---|---|---|---|
| Male | 11 (78.6%) | 18 (75.0%) | 1.000 |
| Age (years) | 70.4 (11.0) | 70.8 (7.3) | 0.760 |
| Lesion size (μm) | 2346 (741) | 3125 (1143) | 0.083 |
| PDT treatments, n | 1.36 (0.63) | 1.96 (0.95) | 0.019 |
| Classic CNV located in subfovea | 6.0 (42.9%) | 21.0 (87.5%) | 0.007 |
| Classic CNV adjacent to polypoidal lesions | 13.0 (92.9%) | 14.0 (58.3%) | 0.030 |
| Increased visual acuity | 8.0 (57.1%) | 4.0 (16.7%) | 0.026 |
PDT, photodynamic therapy; CNV, choroidal neovascularisation.
The lesion size used for PDT of the 38 eyes ranged from 750 to 5400 μm (mean 2838 (1073) μm). The number of PDT treatments that were necessary ranged from 1 to 4 (mean 1.74 (0.89)). The mean lesion size was 2436 (741) μm in the resolved group and 3125 (1143) μm in the persisted group; the lesion size for PDT was not significantly larger in the persisted group than in the resolved group (p = 0.083; table 3). The mean number of PDTs was 1.36 (0.63) in the resolved group and 1.96 (0.95) in the persisted group; the number of PDTs required was thus significantly greater in the persisted group than in the resolved group (p = 0.019; table 3).
Discussion
When eyes with PCV have the appearance of classic CNV on FA, it would be of great help to determine if they do in fact have type 2 CNV, because the visual prognosis is quite different from that of PCV.6,14 However, it is often difficult to discriminate type 2 CNV from pure fibrinous tissue deposition before treatment. In the current study, we investigated eyes with PCV that appeared to have classic CNV, and, based on the reaction to PDT of the subretinal material that corresponded to the classic CNV, we formed two groups: the persisted group and the resolved group. Based on previous histological reports and a recent report on OCT examination, we hypothesise that eyes in the persisted group do indeed have type 2 CNV,15,23 and that eyes in the resolved group have deposition of pure fibrinous tissue, without CNV.15,24 We found no significant difference in pretreatment characteristics, including gender, age, visual acuity or lesion size for PDT between the two groups. However, the number of required PDTs was much lower and final visual acuity was significantly better in the resolved group than in the persisted group. Therefore, eyes with type 2 CNV were considered to have a worse visual prognosis in spite of requiring of a greater number of re‐treatments.
It has been reported that PCV originates from an abnormality of the inner choroidal vessels.1,2,3,10 As most components of PCV are located beneath the RPE, they appear usually as occult CNV on FA,11,12,13 although they sometimes have the appearance of classic CNV on FA. It has been reported that 9–25% of eyes with PCV show classic CNV on FA.1,7,10,14 In a previous report by Spaide et al, classic CNV developed during the follow‐up period in three (25%) of 12 eyes with PCV.1 Previously, Sho et al reported that a cluster of grapelike polypoidal lesions was observed in 10 (9%) of 110 eyes with PCV, and that there was a clear association with classic CNV.14 We found that 38 eyes with PCV showed classic CNV, so eyes with PCV that have the appearance of classic CNV are not uncommon.
Histologically, Gass reported two types of CNV in AMD: type 1 and type 2 CNV.21 In his report, type 1 CNV remained beneath the RPE, while type 2 CNV penetrated the RPE and proceeded into the subretinal space. On FA, type 1 CNV is often seen as occult CNV, while most type 2 CNV appears as the classic CNV.13 Previous histological examinations of surgical specimens from eyes with PCV showed fibrovascular tissue located within Bruch's membrane, with secondary CNV in the subretinal space.16,17 Therefore, some eyes with PCV develop type 2 CNV and show the appearance of classic CNV.
In rare cases, type 2 CNV disappears spontaneously. Treatment with PDT often decreases the amount of leakage from CNV, but an atrophic vascular membrane is often seen on funduscopic examination and OCT imaging, resulting in poor visual recovery.23 In 24 of the 38 eyes of our patients, subretinal material detected with OCT did not resolve completely after PDT. We think that these eyes had type 2 CNV before treatment. However, in the other 14 eyes of our patients, subretinal material detected with OCT disappeared completely after PDT, in accordance with the disappearance of classic CNV. Recently, Otsuji et al reported that in eyes with PCV and apparently classic CNV, the subretinal material just over the polypoidal lesion sometimes consisted of pure fibrinous tissue with no evidence of CNV.15 Leakage from active polypoidal lesions can cause fibrin deposition in the subretinal space, an appearance that may mimic classic CNV on FA.
In the last few years, a number of reports have shown encouraging results of PDT for treatment of subfoveal PCV.12,25,26,27,28 In a recent prospective study by Chan et al, PDT resulted in stable or improved vision in 21 (95%) of 22 eyes with PCV.27 In their report, eyes showing CNV on clinical examination or on FA were excluded from the study. In another study, Silva et al showed that visual acuity improved in 57.1% and was unchanged in 23.8% of eyes with PCV at one year after PDT.12 All eyes had shown occult CNV before treatment. To date, little information is available on the efficacy of PDT in eyes with PCV and type 2 CNV. In the study described herein, visual acuity improved in 16.7% and remained unchanged in 58.3% of eyes after PDT in the persisted group. Treatment effect would be expected to be limited in PCV eyes with type 2 CNV.
In the current study, we investigated the location of the subretinal material that corresponded to classic CNV. The subretinal material was subfoveal in six (42.9%) of 14 eyes in the resolved group, and in 21 (87.5%) of 24 eyes in the persisted group (p = 0.007). In 21 (77.8%) of 27 eyes, therefore, the subretinal material located in the subfovea did not resolve after treatment. In addition, the subretinal material was located adjacent to polypoidal lesions in 13 (92.9%) of 14 eyes in the resolved group, and in 14 (58.3%) of 24 eyes in the persisted group (p = 0.030). In other words, 10 (91%) of 11 eyes in which the subretinal material was separate from the polypoidal lesion showed persistence of the subretinal material after PDT. Therefore, when subretinal material is subfoveal or separate from the polypoidal lesions, it can be hypothesised that the eye actually has type 2 CNV.
In conclusion, true type 2 CNV is not a rare complication of PCV, and the eyes often show what appears to be classic CNV on FA. However, it is difficult to discriminate type 2 CNV from pure fibrinous tissue deposition before treatment, even with a detailed examination by OCT. When subretinal material is seen in the subfoveal region or is separate from the polypoidal lesions, the eye may actually have type 2 CNV, and may thus need to be treated promptly.
Abbreviations
CNV - choroidal neovascularisation
FA - fluorescein angiography
OCT - optical coherence tomography
PCV - polypoidal choroidal vasculopathy
PDT - photodynamic therapy
RPE - retinal pigment epithelium
Footnotes
Competing interests: None.
References
- 1.Spaide R F, Yannuzzi L A, Slakter J S.et al Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 199515100–110. [DOI] [PubMed] [Google Scholar]
- 2.Yannuzzi L A, Ciardella A, Spaide R F.et al The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997115478–485. [DOI] [PubMed] [Google Scholar]
- 3.Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol 200589602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uyama M, Matsubara T, Fukushima I.et al Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 19991171035–1042. [DOI] [PubMed] [Google Scholar]
- 5.Uyama M, Wada M, Nagai Y.et al Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002133639–648. [DOI] [PubMed] [Google Scholar]
- 6.Yannuzzi L A, Wong D W, Sforzolini B S.et al Polypoidal choroidal vasculopathy and neovascularized age‐related macular degeneration. Arch Ophthalmol 19991171503–1510. [DOI] [PubMed] [Google Scholar]
- 7.Moorthy R S, Lyon A T, Rabb M F.et al Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology 19981051380–1385. [DOI] [PubMed] [Google Scholar]
- 8.Costa R A, Navajas E V, Farah M E.et al Polypoidal choroidal vasculopathy: angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res 200524560–586. [DOI] [PubMed] [Google Scholar]
- 9.Ciardella A P, Donsoff I M, Huang S J.et al Polypoidal choroidal vasculopathy. Surv Ophthalmol 20044925–37. [DOI] [PubMed] [Google Scholar]
- 10.Yannuzzi L A, Sorenson J, Spaide R F.et al Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990101–8. [PubMed] [Google Scholar]
- 11.Lafaut B A, Leys A M, Snyers B.et al Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol 2000238752–759. [DOI] [PubMed] [Google Scholar]
- 12.Silva R M, Figueira J, Cachulo M L.et al Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol 2005243973–979. [DOI] [PubMed] [Google Scholar]
- 13.Macular Photocoagulation Study Group Subfoveal neovascular lesions in age‐related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol 19911091242–1257. [PubMed] [Google Scholar]
- 14.Sho K, Takahashi K, Yamada H.et al Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 20031211392–1396. [DOI] [PubMed] [Google Scholar]
- 15.Otsuji T, Tsumura A, Takahashi K.et al Evaluation of cases of polypoidal choroidal vasculopathy showing classic choroidal neovascularisation in their natural course. J Jpn Ophthalmol Soc 2006110454–461. [PubMed] [Google Scholar]
- 16.MacCumber M W, Dastgheib K, Bressler N M.et al Clinicopathologic correlation of the multiple recurrent serosanguineous retinal pigment epithelial detachments syndrome. Retina 199414143–152. [DOI] [PubMed] [Google Scholar]
- 17.Kuroiwa S, Tateiwa H, Hisatomi T.et al Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Experiment Ophthalmol 200432297–302. [DOI] [PubMed] [Google Scholar]
- 18.Shiraga F, Matsuo T, Yokoe S.et al Surgical treatment of submacular haemorrhage associated with idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 1999128147–154. [DOI] [PubMed] [Google Scholar]
- 19.Terasaki H, Miyake Y, Suzuki T.et al Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol 200286321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima M, Yuzawa M, Shimada H.et al Correlation between indocyanine green angiographic findings and histopathology of polypoidal choroidal vasculopathy. Jpn J Ophthalmol 200448249–255. [DOI] [PubMed] [Google Scholar]
- 21.Gass J D. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 1994118285–298. [PubMed] [Google Scholar]
- 22.Hee M R, Baumal C R, Puliafito C A.et al Optical coherence tomography of age‐related macular degeneration and choroidal neovascularisation. Ophthalmology 19961031260–1270. [DOI] [PubMed] [Google Scholar]
- 23.Rogers A H, Martidis A, Greenberg P B.et al Optical coherence tomography findings following photodynamic therapy of choroidal neovascularisation. Am J Ophthalmol 2002134566–576. [DOI] [PubMed] [Google Scholar]
- 24.Iida T, Hagimura N, Sato T.et al Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol 200012916–20. [DOI] [PubMed] [Google Scholar]
- 25.Quaranta M, Mauget‐Faysse M, Coscas G. Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Am J Ophthalmol 2002134277–280. [DOI] [PubMed] [Google Scholar]
- 26.Spaide R F, Donsoff I, Lam D L.et al Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina 200222529–535. [DOI] [PubMed] [Google Scholar]
- 27.Chan W M, Lam D S, Lai T Y.et al Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one‐year results of a prospective case series. Ophthalmology 20041111576–1584. [DOI] [PubMed] [Google Scholar]
- 28.Lee S C, Seong Y S, Kim S S.et al Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica 2004218193–201. [DOI] [PubMed] [Google Scholar]
- 29.Sasahara M, Tsujikawa A, Musashi K.et al Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol 2006142601–607. [DOI] [PubMed] [Google Scholar]