Abstract
Background
Visceral hypersensitivity is an important pathophysiological factor in irritable bowel syndrome (IBS). Pre‐clinical studies suggest that the α2δ ligand pregabalin reduces both visceral allodynia and hyperalgesia, but is inactive on basal sensitivity.
Aim
To assess the effect of pregabalin on the perception of rectal distension in hypersensitive IBS patients.
Methods
Twenty‐six patients with Rome‐II‐defined IBS (aged 18–46 years, 7 male) were included in a randomized, double‐blind, placebo‐controlled, parallel‐group study in which they received either 3 weeks oral pregabalin (titrated: 50 mg tid days 1–3, 100 mg tid days 4–7, 150 mg tid days 8–11; fixed 200 mg tid days 12–21 ±4) or placebo control. Rectal sensitivity was assessed using a barostat technique, in which sensory thresholds were determined using the ascending method of limits, followed by tracking both before and after treatment. Only patients with a pain threshold of ⩽28 mmHg were included in the study.
Results
Pregabalin significantly increased the sensory thresholds from baseline for first sensation (p = 0.045), desire to defecate (p = 0.008) and pain (p = 0.048) compared with placebo control. In addition, pregabalin significantly increased rectal compliance (p<0.0001), although this appeared to be unrelated to the changes in sensitivity. Despite the occurrence of mild dizziness and somnolence, pregabalin was generally well tolerated.
Conclusions
Pregabalin increased distension sensory thresholds to normal levels in IBS patients with rectal hypersensitivity. A concomitant increase in rectal compliance appeared to be unrelated to the reduction in sensitivity. These data suggest that α2δ ligands are worthy of further investigation in the treatment of visceral pain disorders, including IBS.
Keywords: pregabalin, α2δ ligands, visceral sensitivity, irritable bowel syndrome
Since the early 1970s, studies have shown that patients with irritable bowel syndrome (IBS) have increased perception of balloon distension of the colon and rectum when compared with healthy volunteers.1,2 Other studies have reported similar observations in the jejunum,3 ileum4 and oesophagus,5,6 and we have shown mean pain thresholds to be significantly reduced along the entire length of the gastrointestinal tract in IBS patients when compared with healthy volunteers.7,8 Furthermore, comparison of each patient's pain threshold with the 95% normal reference range revealed that more than 90% of patients exhibited hypersensitivity in at least one of either the oesophagus, duodenum, jejunum, ileum, colon or rectum.7,8 Hypersensitivity has also been shown to be associated with a wider pattern of referred somatic pain in IBS patients compared with healthy controls,9,10,11 suggesting altered processing within the central nervous system (CNS). These observations have led many investigators to consider hypersensitivity to be a determinant and possibly a biological measure of IBS,9,12 thus providing a rationale for the development of novel therapies aimed at modulating sensory neurotransmission from the intestine.
Pregabalin (Lyrica), is a second‐generation α2δ ligand that has recently gained approval in Europe for the treatment of neuropathic pain and epilepsy, and in the USA for the management of neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia and as an adjunctive therapy for adults with partial‐onset seizures.13,14 It is 2–10 times more potent, possesses more linear pharmacokinetics and, consequently, has more predictable pharmacological effects than the prototype α2δ ligand, gabapentin.13,14 Although it is structurally related to γ‐aminobutyric acid (GABA), a major inhibitory neurotransmitter in the CNS, it is functionally unrelated and inactive at GABAA, GABAB or benzodiazapine receptors, and is not converted metabolically into GABA or a GABA agonist.13,14 Furthermore, clinically effective concentrations of pregabalin have been shown to have no effect on GABA uptake or degradation.13,14 Despite incomplete knowledge concerning the precise mechanism of action of pregabalin, it is believed to bind potently to the α2δ auxiliary protein associated with voltage‐gated calcium channels, reducing depolarization‐induced calcium influx at the nerve terminals, and consequently the release of several excitatory neurotransmitters, including glutamate, noradrenaline, substance P and calcitonin gene‐related peptide (CGRP).13,14
Pregabalin has been shown to be effective in several animal models of inflammatory and neuropathic pain. For example, it has been shown to block both thermal and mechanical hyperalgesia induced by inflammatory, surgical and nerve injuries15,16,17,18,19,20 and to inhibit both the static and dynamic components of mechanical allodynia induced by streptozocin.21 Morphine and amitriptyline have been shown to only inhibit the static component of allodynia, suggesting superior anti‐allodynic activity for pregabalin.21 Furthermore, in animal models of visceral pain, pregabalin has been shown to dose‐dependently reduce trinitrobenzene‐sulfonic‐acid‐induced colonic allodynia22 and to suppress lipopolysaccharide (LPS)‐induced hyperalgesia, measured as a reduction in the amount of abdominal contractility in response to rectal distension in LPS‐treated animals23 but to be inactive on basal sensitivity.22,23 Thus, pregabalin appears to have a broad spectrum of anti‐hyperalgesia activity in diverse animal models and is therefore an appropriate candidate for assessment in clinical conditions that are characterized by hyperalgesia.
The aim of the present study was to assess the effect of pregabalin on the perception of rectal distension in IBS patients with rectal hypersensitivity.
Materials and methods
Patients
Forty‐one patients with Rome‐II‐defined IBS24 (28 female, 13 male) aged between 18 and 46 years were enrolled. Patients were recruited from the hospital's out‐patient departments (tertiary patients excluded), local general practices, advertisements in regional newspapers and from a departmental volunteer pool of patients. No patient had coexistent disease other than IBS and all had normal biochemistry, haematology, urinalysis and normal colonoscopy/sigmoidoscopy within 5 years of screening. Patients were excluded if they had either: severe constipation (one stool every seventh day or less frequently) or diarrhoea (⩾7 stools/day); history of gastrointestinal surgery (other than appendicectomy, benign polypectomy, hiatus hernia repair or cholecystectomy, as long as the IBS symptoms did not start within 1 year of surgery); current evidence or history of laxative abuse; concomitant psychiatric diagnosis; had gastrointestinal symptoms related to or exacerbated by the consumption of milk or milk products; drank above the recommended safe alcohol limit (females <14 units/week; males <21 units/week); had participated in a trial of any drug within 30 days prior to visit 1; or were taking drugs that might modify gastrointestinal function. Females were also excluded if they were pregnant or breastfeeding, and females of child‐bearing potential had to be using adequate contraception (including a barrier or hormonal method) and have a confirmed negative serum pregnancy test at screening. Although menstrual status was not formally noted in this study, we avoided assessment of visceral sensitivity at the time of menses, as previous studies have shown visceral sensitivity is increased at this time in IBS patients.25 Moreover, patients were asked to abstain from smoking, caffeine and alcohol for 24 hours prior to visits 2 and 3. The study was approved by South Manchester Medical Research Ethics Committee and all subjects gave written informed consent.
Study design and procedure
The study was conducted between 5 February 2002 and 24 September 2003, and was single‐centre, randomized, double‐blind, placebo‐controlled, parallel‐group in design, in which rectally hypersensitive IBS patients received either 3 weeks oral pregabalin or matched placebo control. Pregabalin was titrated in a blinded fashion over the first 11 days, such that during days 1–3 they received 50 mg tid; during days 4–7, they received 100 mg tid; and during days 8–11, they received 150 mg tid. They then received fixed dosing during days 12–21 ±4 days of 200 mg tid. The fixed final 600 mg/day dose of pregabalin was selected because it represents the maximum approved treatment dose and therefore provided optimal conditions to identify potential effects on visceral sensation. Randomization was performed within the Pharmacy Department of Wythenshawe Hospital according to a computer‐generated randomization schedule provided by Pfizer. Neither the patients nor the study investigators, including personnel from Pharmacy, were aware of the treatment assignment.
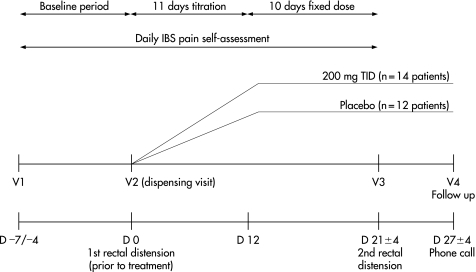
Figure 1 shows a schematic diagram of the protocol used. All patients completed a baseline period of 4–7 days in which they scored the severity of their abdominal pain every evening using a Likert scale of 0–10 (“Daily IBS Pain Self‐Assessment”, where 0 = “no pain” and 10 = “worse possible pain”). Following the baseline period, patients underwent rectal sensitivity assessment using a barostat technique, in which sensory thresholds were determined using the ascending method of limits followed by tracking (day 0 (D 0)). Patients with a pain threshold ⩽28 mmHg and who had completed a record of abdominal pain for at least 4 days in the “Daily IBS Pain Self‐Assessment” diary were then randomized to treatment and asked to start medication on day 1 and continue dosing until their return on day 21 ±4, when they underwent their second rectal sensitivity assessment. Just prior to the second sensitivity assessment, a 5 ml blood sample was taken for later determination of plasma pregabalin concentration using a validated HPLC‐UV method. Throughout the experimental period, patients were asked to continue to record their pain severity using the “Daily IBS Pain Self‐Assessment” diary. All patients were followed up by telephone on day 27 ±4, and were asked to report any adverse events as either mild, moderate or severe on day 7 following start of treatment (by telephone), on visit 3 (second rectal sensitivity) and at follow‐up.
Figure 1 Schematic diagram of protocol used. Note that pregabalin was titrated in blinded fashion over the first 11 days, such that during days 1–3 they received 50 mg tid, during days 4–7 they received 100 mg tid; and during days 8–11 they received 150 mg tid. During days 12–21 ±4 days, the dose was fixed at 200 mg tid.
Rectal barostat
All patients presented to the Neurogastroenterology Unit after fasting for at least 10 hours. Following bowel preparation (Fleet® phosphate enema), a catheter (customized rectal barostat catheter, part no. C7‐2CB‐R‐22F; MUI Scientific, Mississauga, Ontario, Canada), to which was attached a polyethylene bag (Pillow Type Rectal Barostat Balloon, part no. CT‐BP600R; length, 22 cm: diameter, 15 cm; capacity 600 ml; MUI Scientific), was inserted into the rectum so that the middle of the balloon was located approximately 10 cm from the anal verge. To minimalize the effects of the abdominal viscera on the balloon volume, the patients were placed in a semi‐prone position with the foot of the bed elevated by 15 degrees. The bag was then unfolded by inflating it transiently with 150 ml of air and then deflating it completely. Following a rest period and 1 hour after the enema, the catheter was connected to a barostat (G&J Electronics Inc., Toronto, Ontario, Canada) and the pressure in the bag increased from 6 mmHg in steps of 2 mmHg until respiratory excursions were observed. The basal operating pressure (BOP) was defined as 2 mmHg above the minimal distension pressure at which respiratory excursions were clearly recorded from the barostat tracing.
An initial “conditioning” distension of the rectum was then performed in which pressure was increased from 0 mmHg in steps of 4 mmHg for 15 seconds per step until 20 mmHg was reached. Previous studies have shown that an initial “conditioning” distension to 20 mmHg renders subsequent assessments of compliance and perception more reproducible.26 The bag was then deflated to BOP before proceeding to the ascending method of limits.
Rectal compliance and sensory thresholds were measured by stepwise inflation, starting at BOP and increasing in steps of 2 mmHg for 2 minutes, with a return to BOP between steps for 2 minutes, up to a maximum pressure of 48 mmHg. One minute after commencement of each inflation, patients were asked to assess feelings of “Stool Sensation” and “Pain/Discomfort” using the following scales: (i) “Stool Sensation Scale”, where 0 = no sensation, 1 = first sensation, 2 = constant sensation/gas, 3 = feeling of a need to defecate, and 4 = urgent need to defecate; and (ii) “Pain/Discomfort Scale”, where 0 = no pain/discomfort, 1 = mild but not sustained pain/discomfort, 2 = mild but sustained pain/discomfort, 3 = moderate pain, and 4 = intense pain. Inflations were continued until the patient's first perceived moderate pain (score 3 on the Pain/Discomfort Scale) or on reaching a maximum pressure of 48 mmHg, at which point moderate pain was arbitrarily set at 48 mmHg.
Tracking commenced at the first perception of moderate pain (score 3).27 This involves subsequent distensions being adjusted up or down depending on the patient's response to the previous distension. For example, if moderate pain was reported on the previous trial, the next distension was decreased by 2 mmHg or kept the same, whereas if the patient reported no or less than moderate pain, the next inflation was increased by 2 mmHg or kept the same. In order to make the changes in amount of distension unpredictable, a random numbers table was used to decide whether to increase/decrease the distension or keep it the same on the next trial. Tracking continued up to a total of 18 distensions or after reaching the upper limit of 48 mmHg. At any time, the patient or investigator could discontinue the distension session by pressing the “panic button”, which automatically and permanently deflates the bag.
Data analysis
The following measurements were derived: (i) the sensory thresholds for “first sensation” (score 1 on the “Stool Sensation Scale”) and the “feeling of a need to defecate” (score 3 on the “Stool Sensation Scale”) during the ascending method of limits, (ii) the sensory threshold for moderate pain defined as the average pressure over the series of trials tracked up to a total of 18 distensions or after reaching the upper limit of 48 mmHg, and (iii) rectal compliance.
The rectal volume–pressure relationship was examined during the ascending method of limits and up to commencement of tracking (first moderate painful sensation). The volume–pressure relationship was examined by fitting a sigmoidal emax model.28 The volume–pressure relationship during ramp distension is nonlinear. Therefore, a linear model may not detect differences between different segments of the compliance curve. The sigmoidal emax model is an appropriate fit to this data and allows treatment groups to be compared by examining the slopes (steepness of the curves).
In addition, the average pain severity score was calculated before and after treatment.
Statistical considerations
The sample size for this exploratory study was not based on rigorous statistical considerations; however, with 12 patients per treatment group, the trial had an 80% power to detect at the 0.05 level (2‐sided) a standardized difference of 1.2, which corresponds to a major treatment effect.
The primary variable of interest in the study was the pressure threshold for moderate pain. All other variables of pressure threshold for first sensation, pressure threshold for feeling of a need to defecate, compliance and abdominal pain severity were secondary endpoints. For each variable, a two‐sided test at the 5% level of significance was applied. As the secondary endpoints are exploratory in nature, no adjustment for multiplicity was applied. The hypothesis of interest was: To compare the change from baseline for each sensory threshold following pregabalin and placebo treatment using the Wilcoxon rank sum test.29 The magnitude of the effect was estimated using the corresponding Hodges–Lehmann estimate.29 This procedure is more robust to departures from normality and outliers than the t‐test. Post‐treatment compliance was assessed following pregabalin and placebo treatments using appropriate non‐linear regression modelling. Initially, a simple emax model (nonlinear) was applied. The model was built up sequentially in terms of complexity and the difference in the –2× log likelihood was used to compare models in order to select the most appropriate final model. An appropriate model that described the volume–pressure relationship was the sigmoidal emax model:28 Volume = alpha + {delta × pressuregamma}/{betagamma + pressuregamma}, where alpha = E0 + eta1; beta = PE50 + eta2; delta = Vmax + eta3; E0 is the estimate volume when the pressure is zero (Eta1 allows for between subject variability around E0); PE50 is the pressure that is half of the maximum possible increase in volume (Eta2 allows for between‐subject variability around the PE50); Vmax is the maximum possible increase in volume from E0 with increasing pressure (the volume asymptotes to (E0 plus Vmax) with increasing pressure. Eta3 allows for between‐subject variability around Vmax); gamma P and T describes the steepness of the curve around PE50 for the placebo (ie the slope of the placebo volume– pressure curve) and treatment groups, respectively.
The difference between the log likelihood of this model (which estimates separate slopes for each treatment) and the same model with a common slope was compared to a chi square 1df. This was used to derive a p value for the statistical significance of treatment on volume–pressure slopes.
The model was fitted in PROC NLMIXED in SAS.30 The mixed effects modelling allowed for patients having different E0, PE5− and Vmax. The residuals were checked to confirm that the model fit was appropriate.
The SAS statistical package was used in all data analysis.30
Results
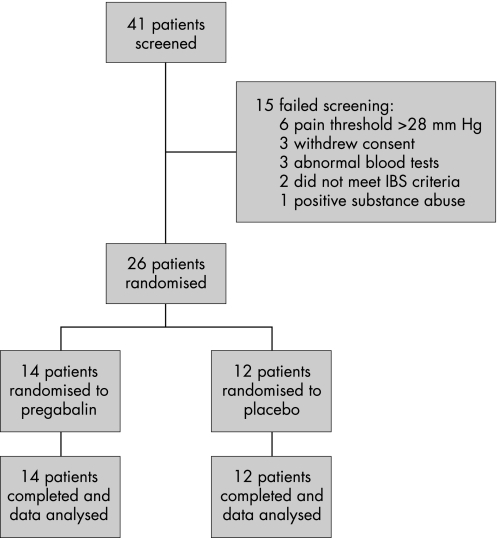
Of the 41 patients enrolled, 12 failed screening and 3 withdrew consent (figure 2). Of the 12 patients who failed screening, 6 had baseline pain thresholds above 28 mmHg, 3 had abnormal blood tests, 1 proved positive for substances of abuse and 2 failed to meet study criteria for IBS (eg too severe bowel habit abnormality). Of the remaining patients, 14 were randomized to pregabalin (aged 18–46 years, 11 female) and 12 to placebo (aged 21–41 years, 8 female).
Figure 2 Study flow diagram.
Sensory thresholds
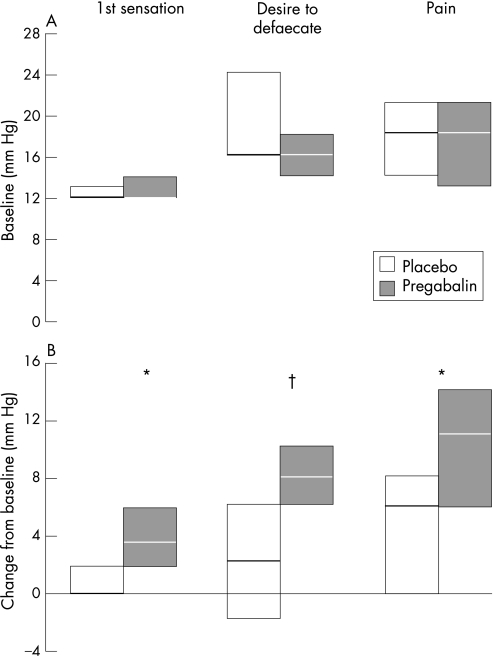
Under baseline conditions (D 0), there was no difference in sensory thresholds for first sensation, desire to defecate and moderate pain between patients randomized to receive pregabalin and placebo control (figure 3a). Following treatment, the change from baseline for first sensation (median difference pregabalin – placebo: 2.0 mmHg (95% CI = 0, 4.0; p = 0.045); desire to defecate (6.0 mmHg (2.0, 10.0); p = 0.008) and pain (5.4 mmHg (0.1, 11.3); p = 0.047) were all significantly greater for those patients who had received pregabalin compared with placebo control (figure 3b).
Figure 3 Baseline (A) and change from baseline (B) pressure thresholds to cause first sensation, desire to defecate and pain for IBS patients treated with pregabalin or placebo control. Note thresholds to cause first sensation and desire to defecate were obtained from the ascending method of limits, whereas the sensory threshold to cause pain was obtained from the tracking technique. Data expressed as median and IQR. *p<0.05, †p<0.01 compared with placebo control.
Compliance
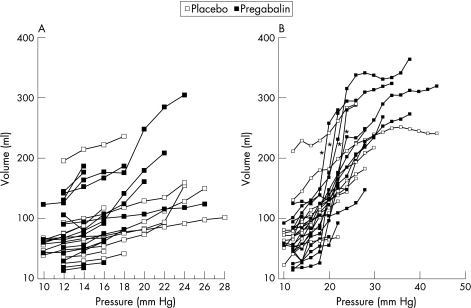
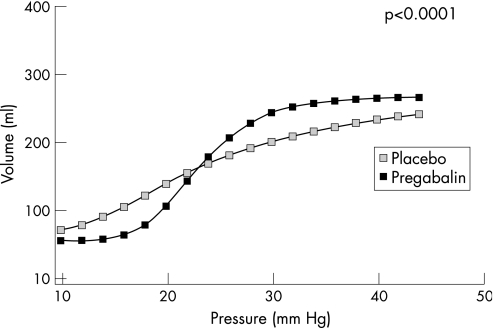
Under baseline conditions (D 0), there was no difference in compliance between patients randomized to receive pregabalin and placebo control (figure 4A). Following treatment (day 21 ±4), the emax model predicted that the slope of the volume–pressure curve for pregabalin (8.379 (95% CI = 7.266, 9.491) was significantly steeper than that for placebo control (3.099 (2.294, 3.905); p<0.001) (figure 4B and 5). Four patients had particularly steep volume–pressure relationships (Figure 4B, indicated by asterisks) following pregabalin treatment; however, their change in pain threshold from baseline appeared not to be particularly greater than that of the rest of the patients (ie 1.6 mmHg, 8.6 mmHg, 9.1 mmHg and 13.1 mmHg compared with a median change of 10.6 mmHg, range 1.5–33.6 mmHg).
Figure 4 Line diagrams showing individual patient data for volume plotted against pressure pre‐ (baseline) (A) and post‐ (B) treatment. Note slopes are similar under baseline conditions, but steeper between the pressures of 18 to 25 mmHg following treatment with pregabalin compared with placebo control. Patients indicated by asterisks are those with particularly increased rectal compliance following pregabalin treatment (see figures 3 and 6).
Figure 5 Sigmoidal emax model of volume–pressure relationship following treatment with pregabalin or placebo control. Note slope of volume–pressure curve is 1.96 steeper for pregabalin compared with placebo (95% CI = 1.50–2.41; p<0.0001).
Symptoms
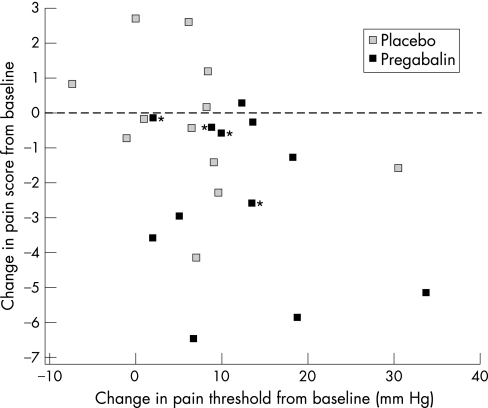
There was no statistical difference in the baseline average daily pain scores between patients randomized to receive pregabalin (5.0 (3.86, 6.29), median (IQR)) or placebo (3.57 (2.86, 4.57)). However, following treatment, there was a tendency for the average daily pain score to decrease in patients receiving pregabalin compared with placebo (median difference pregabalin‐placebo: −1.79 (95% CI = −3.86, 0.143); p = 0.068). This reflected more patients reporting an improvement in their pain score (score<0, with some patients reporting <−5) with pregabalin compared with placebo (figure 6). Furthermore, the improvement in average pain score appeared to increase as the pain threshold increased in patients receiving pregabalin, although the presence of one influential outlier patient, with a change in pain threshold of >30 mmHg, and the small numbers of patients studied, prevented a functional relationship between the two to be firmly established (figure 6). Identification of the four patients who had steep volume–pressure relationships (figure 4B, indicated by asterisks) following pregabalin treatment showed them to have no greater improvement in their symptom of pain than the remainder of the patients (figure 6, indicated by asterisks).
Figure 6 Relationship between change in pain threshold and change in average daily pain score from baseline for IBS patients treated with pregabalin and placebo control. Note that more patients receiving pregabalin exhibited an improvement in pain (score<0) than placebo and that the pain of some patients markedly improved (score<−5). Patients indicated by an asterisk are those with the greatest change in rectal compliance following pregabalin treatment (see figure 4B).
Plasma concentrations
Following treatment, the plasma concentration of pregabalin was 8.624 ±4.255 μg/ml (mean ±SD). The change in sensory threshold did not appear to correlate with the plasma concentration of pregabalin.
Adverse events
Table 1 lists the adverse events reported during treatment with pregabalin and placebo control. A similar total number of adverse events were reported by both groups, although those treated with pregabalin reported more adverse events associated with the nervous system (12 vs 4 patients), but less with the body as a whole (1 vs 9 patients) compared with those treated with placebo (table 1). The most frequently reported adverse events were dizziness (10 patients) and somnolence (5 patients) during pregabalin treatment, and headache (8 patients) and abdominal pain (3 patients) during placebo treatment (table 1). The dizziness and/or somnolence reported during pregabalin treatment was often intermittent and lasted over a median of 11 days (range, 0–33 days) starting during the 50 mg dose in 7 patients, 100 mg dose in 3 patients and 150 mg dose in 2 patients. On the day of the post‐pregabalin visceral sensitivity test, 5 of the 14 patients reported dizziness and/or somnolence. There was no difference in the change in pain threshold from baseline between subjects experiencing these side effects and those without side effects (median difference: 0.10 mmHg (95% CI = −11.50 to 8.40)). Overall, 75% of adverse events were reported as mild, 17% moderate and 8% severe, and all the severe adverse events were reported by the same patient following pregabalin.
Table 1 Number of rectally hypersensitive IBS patients reporting the most commonly reported adverse events following treatment with pregabalin and placebo control.
| Pregabalin (n = 14) | Placebo (n = 12) | |
|---|---|---|
| Body as a whole | 1 | 9 |
| Accidental injury | 0 | 1 |
| Asthenia | 1 | 0 |
| Headache | 0 | 8 |
| Infection | 0 | 2 |
| Malaise | 1 | 0 |
| Pelvic pain | 0 | 1 |
| Digestive | 4 | 5 |
| Abdominal pain | 0 | 3 |
| Anorexia | 0 | 1 |
| Constipation | 3 | 2 |
| Flatulence | 0 | 2 |
| Nausea | 1 | 1 |
| Vomiting | 1 | 0 |
| Nervous system | 12 | 4 |
| Amnesia | 1 | 0 |
| Confusion | 1 | 0 |
| Dizziness | 10 | 1 |
| Euphoria | 1 | 0 |
| In‐coordination | 3 | 0 |
| Insomnia | 0 | 1 |
| Somnolence | 5 | 2 |
| Speech disorder | 1 | 0 |
| Thinking difficulty | 2 | 0 |
| Respiratory | 1 | 1 |
| Cough increase | 1 | 0 |
| Pharyngitis | 1 | 0 |
| Sinusitis | 0 | 1 |
| Skin and appendages | 0 | 1 |
| Sweating | 0 | 1 |
| Special senses | 3 | 1 |
| Amblyopia | 2 | 1 |
| Eye haemorrhage | 1 | 0 |
| Urogenital | 0 | 1 |
| Polyuria | 0 | 1 |
Discussion
This is the first study to assess the effect of a new member of a therapeutic class, known as α2δ ligands, on visceral sensation in humans. Specifically, our study has shown that the second‐generation α2δ ligand pregabalin significantly increases rectal sensory thresholds to distension in hypersensitive IBS patients. Thus, these ligands might provide a promising new approach to treating this condition.
Previous studies within our unit using a similar distension protocol have shown that the mean pressure threshold to cause moderate pain in healthy volunteers is approximately 31 mmHg, with 2.5th and 97.5th percentile limits of 24 and 38 mmHg, respectively.31 Although the protocol targeted patients for this particular study who had pain thresholds of ⩽28 mmHg rather than <24 mmHg in order to better facilitate patient recruitment, examination of figure 3A shows that the median pain thresholds for both groups of patients were well below the 2.5th percentile limit for healthy volunteers (pregabalin 17.7 mmHg vs placebo 17.9 mmHg), indicating that both groups were “hypersensitive” under baseline conditions. Post‐treatment, the pain thresholds increased in both groups (figure 3B); however, only in the pregabalin‐treated patients did the median pain threshold (ie 29.1 mmHg) normalize to the mean for healthy volunteers. The median pain threshold for those treated with placebo (21.4 mmHg) rose to just below the 2.5th percentile limit for healthy volunteers, indicating that this group remained hypersensitive post‐treatment. The observation that pregabalin appears to normalize rather than desensitize (ie make hyposensitive) the perception of rectal distension in hypersensitive patients supports the findings in animals that pregabalin modulates the “hyper‐” but not the “normal” sensitivity state;22,23 although studies in normally sensitive subjects, such as healthy volunteers, would be required to confirm this assumption. As well as the anti‐allodynic activity of pregabalin, we also noted an increase in the sensory thresholds for first sensation and desire to defecate, highlighting its concomitant visceral anti‐hyperalgesic properties. Such qualities, if confirmed by larger studies, would be considered to be most desirable for any new pharmacological intervention being developed for the treatment of functional bowel disorders.
Measurement of rectal compliance was particularly difficult under baseline conditions as the hypersensitive state of the patients prevented much of the volume–pressure relationship from being determined. However, application of a nonlinear emax model to the post‐treatment volume–pressure data predicted that rectal compliance was significantly greater in those treated with pregabalin compared with placebo control. This was the case even when the four patients with the steepest slopes were excluded from the analysis, with the emax model estimating the slope of the volume–pressure curves from subjects treated with pregabalin to be 7.22 and that of the volume–pressure curves from subjects treated with placebo to be 2.99 (p<0.05), which is similar to the full data analysis set and confirms that these four patients with the steepest slopes were not solely driving the results. The fitted emax model assumes a common underlying curve to all the data (including pregabalin pressures). The model, although allowing for between‐patient variability, estimates maximum and minimum effects and the P50; therefore, it is a reliable model for detecting whether slopes are statistically different. Moreover, the model aims to estimate the true and unknown slope of the pressure relationship. Thus, although it is true that more data on larger pressures (eg greater than 28 mmHg), especially for the placebo group, would have been more informative, the emax model can fit an appropriate curve to this data to estimate the true slope. However, it must be noted that, from the shape of the summary compliance curves (figure 5), there is also a possibility that pregabalin may have shifted the volume–pressure curve at lower pressures to the right (ie indicative of a change in tension), which while not assessable using such modelling techniques, may have further affected the volume–pressure relationship and, consequently, possibly sensitivity. Although using such a modelling technique prevents correlation with rectal sensitivity in individual patients, the four patients who had steep volume–pressure relationships (ie greater compliance) had no greater increases in pressure thresholds for pain following pregabalin compared with the rest of the group, suggesting that these two findings may be independent of each other. This is supported by the previous observations that drug‐induced gastric relaxation (ie increased compliance) is not always associated with a reduction in pain perception, as shown by both clonidine and nitroglycerin increasing gastric compliance but only clonidine reducing pain perception.32 However, at present, the data are too limited, especially at higher pressure levels, to make any conclusive statements about the effects of pregabalin on rectal compliance and tension, and the consequent influence on visceral sensation.
As well as the effects on somatic and visceral pain, pregabalin has been shown to exhibit anxiolytic properties in both animals and humans,13,33,34,35 which might be expected to reduce the patient's vigilance to the distension procedure and, consequently, may lead to an increase in pain thresholds. However, although anxiety levels were not directly assessed, we believe this to be unlikely in this study as the effects of vigilance were minimized by the use of a tracking distension protocol, which makes the pressure inflations unpredictable to the subject. Furthermore, our observations that the sensory thresholds for first sensation and desire to defecate, two sensations not normally expected to be under significant psychological influence, were also increased following pregabalin, suggests that the anxiolytic properties of pregabalin33,34,35 were probably not playing a major role in modulating visceral sensation. Indeed, many studies have examined the possible role of psychological factors, such as anxiety on visceral sensation in IBS and, in general, have been unable to show a direct relationship.2,36 In addition, anxiolytic agents such as buspirone have been shown to have no significant effect on colonic sensitivity to balloon distension.37
An additional factor that may have influenced the perceptual response to rectal distension was the dizziness and/or somnolence that many patients experienced during treatment with pregabalin. The dizziness and somnolence, however, tended to be mild and intermittent and had resolved in 9 out of the 14 patients by the day of the post‐treatment barostat study. Moreover, the change in pain threshold from baseline in patients experiencing dizziness and/or somnolence on the day of the post‐treatment barostat study was no different from that seen in those who were not experiencing these adverse events. These observations, along with the fact that normal sensitivity22,23 and the responsiveness to tests of acute pain38 in animals appears to be unaltered by pregabalin, suggests that dizziness and/or somnolence have probably no or little effect on the perceptual response to distension. Further support is provided by the observations that amitryptiline, a drug that also causes somnolence, does not alter perceptual responses to gastric distension in patients with functional dyspeptia, although it has never been studied in an experiment comparable to ours.39 Thus, while we cannot rule out the central anxiolytic and side‐effect profile (eg dizziness and/or somnolence) of pregabalin as being a possible contributor to the modulation in visceral sensation observed in this study, the possibility remains that the sensory effects of pregabalin result from modulation of visceral afferent‐nerve function, although the precise mode of action in humans is still to be determined. Animal in vitro studies have suggested that following binding of pregabalin to the α2δ subunit of the voltage‐dependent calcium channels, influx of calcium into the presynaptic nerve terminal is reduced. This leads to a reduction in release of excitatory amino acids, substance P and CGRP—which, in animal models, have been shown to modify visceral sensation.13,14 However, the mechanism of modulation of rectal compliance by pregabalin remains to be explored.
Finally, we have also shown a tendency (p = 0.068) for abdominal pain to improve following treatment with pregabalin. However, the significance of this observation in such a limited number of patients cannot be determined until a much larger cohort of patients are evaluated in a controlled clinical trial. Furthermore, whether the improvement in pain was associated with the normalization in visceral sensation cannot be determined from this study, as their correlation was influenced by the presence of an influential outlier patient who had a change in pain threshold of >30 mmHg. Similarly, because of the modelling approach used to analyze the compliance data, no assessment of relationship to symptom improvement could be made, although the four patients who had the steepest volume–pressure relationships (figure 4B, indicated by asterisks) did not appear to have any greater improvement in abdominal pain compared with the rest of the group (Figure 6, indicted by asterisks). However, some studies have suggested an association between symptom improvement and drug‐induced increased gastric compliance in functional dyspepsia patients with impaired postprandial accommodation,40 although others have failed to reproduce these observations.41
In conclusion, pregabalin exhibited several potentially important physiological effects on both rectal sensation and compliance. We therefore suggest that α2δ ligands are worthy of further physiological and clinical investigations for the treatment of diseases affecting both motor and sensory functions of the gastrointestinal tract.
Acknowledgements
This study was kindly funded by Pfizer Global Research and Development, Sandwich, Kent, UK. IJ, DPS and JDG are all employees and stockholders of Pfizer Ltd.
Abbreviations
BOP - basal operating pressure
CGRP - calcitonin gene‐related peptide
CNS - central nervous system
GABA - γ‐aminobutyric acid
IBS - irritable bowel syndrome
LPS - lipopolysaccharide
References
- 1.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 197314125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton L A. Evidence of abnormal rectal sensitivity in IBS. In: Camilleri M, Spiller R, eds. Irritable Bowel Syndrome: Diagnosis and Treatment. Chapter 8. London: WB Saunders, 200269–76.
- 3.Accarino A M, Azpiroz F, Malagelada J ‐ R. Symptomatic responses to stimulation of sensory pathways in the jejunum. Am J Physiol 1992263G673–G677. [DOI] [PubMed] [Google Scholar]
- 4.Kellow J E, Miller L J, Phillips S F.et al Dysmotility of the small intestine in irritable bowel syndrome. Gut 1988291236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costantini M, Sturniolo G C, Zaninotto G.et al Altered esophageal pain threshold in irritable bowel syndrome. Dig Dis Sci 199338206–212. [DOI] [PubMed] [Google Scholar]
- 6.Trimble K C, Farouk R, Pryde A.et al Heightened visceral sensation in functional gastrointestinal disease is not site specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci 1995401607–1613. [DOI] [PubMed] [Google Scholar]
- 7.Francis C Y, Houghton L A, Whorwell P J.et al Enhanced sensitivity of the whole gut in patients with irritable bowel syndrome [abstract]. Gastroenterology 1995108601 [Google Scholar]
- 8.Hammonds R, Houghton L A, Whorwell P J. Urge and no‐urge constipation predominant irritable bowel syndrome (IBS): sensory dysfunction of the whole gut [abstract]. Gastroenterology 2000118(4)830 [Google Scholar]
- 9.Mertz H, Naliboff B, Munakata J.et al Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 199510940–52. [DOI] [PubMed] [Google Scholar]
- 10.Dawson A M. Origin of pain in the irritable bowel syndrome. In: Read NW, ed. Irritable Bowel Syndrome. Philadelphia: Grune and Stratton, 1985155–162.
- 11.Kingham J G, Dawson A M. Origin of chronic right upper quadrant pain. Gut 198526783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouin M, Plourde V, Boivin M.et al Rectal distension testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 20021221771–1777. [DOI] [PubMed] [Google Scholar]
- 13.Huckle R. Pregabalin Pfizer. Curr Opin Invest Drugs 2004582–89. [PubMed] [Google Scholar]
- 14.Ben‐Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 200445(Suppl 6)13–18. [DOI] [PubMed] [Google Scholar]
- 15.Xiao W, Bennett G I. Gabapentin relieves abdominal pains in a rat model of painful peripheral neuropathy [abstract]. Soc Neurosci 199521356 [Google Scholar]
- 16.Field M J, Holloman E F, McCleary S.et al Evaluation of Gabapentin and S‐(+)‐3‐isobutylgaba in a rat model of post‐operative pain. J Pharmacol Exp Ther 19972821242–1246. [PubMed] [Google Scholar]
- 17.Field M J, Oles R J, Lewis A S.et al Gabapentin (neurontin) and S‐(+)‐S‐isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol 19971211513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter J C, Gorgas K R, Hedley L R.et al The effect of novel anti‐epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol 1997324153–160. [DOI] [PubMed] [Google Scholar]
- 19.Hwang J H, Yaksh T L. Effect of subarachnoid gabapentin on tactile‐evoked allodynia in a surgically induced neuropathic model in the rat. Reg Anaesth 199722249–256. [DOI] [PubMed] [Google Scholar]
- 20.Houghton A K, Lu Y, Westlund K N. S‐(+)‐3‐isobutylgaba and its stereoisomer reduces the amount of inflammation and hyperalgesia in an acute arthritis model in the rat. J Pharmacol Exp Ther 1998285533–538. [PubMed] [Google Scholar]
- 21.Field M J, McCleary S, Hughes J.et al Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain 199980391–398. [DOI] [PubMed] [Google Scholar]
- 22.Diop L, Raymond F, Fargeau H.et al Pregabalin (CI‐1008) inhibits the trinitrobenzene sulfonic acid‐induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther 20023021013–1022. [DOI] [PubMed] [Google Scholar]
- 23.Eutamene H, Coelho A M, Theodorou V.et al Antinociceptive effect of pregabalin in septic shock‐induced rectal hypersensitivity in rats. J Pharmacol Exp Ther 2000295162–167. [PubMed] [Google Scholar]
- 24.Thompson W G, Longstreth G F, Drossman D A.et al Functional bowel disorders and functional abdominal pain. Gut 199945(Suppl 11)42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghton L A, Lea R, Jackson N.et al The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut 200250471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammer H F, Phillips S F, Camilleri M.et al Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol 1998274G584–G590. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead W E, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo‐Wellcome Research, UK. Dig Dis Sci 199742223–241. [DOI] [PubMed] [Google Scholar]
- 28.Rowland M, Tozer T N. Pharmacological Responses. In: Clinical Pharmacokinetics: Concepts and Applications. 3rd Edition. Chapter 20. London: Lippincott Williams and Wilkins 1995340–363.
- 29.Sprent P.Nonparametric statistical methods. London: Chapman Hall, 1989
- 30.SAS Institute Inc http://www.sas.com
- 31.Lea R, Houghton L A, Calvert E L.et al Gut‐focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther 200317635–642. [DOI] [PubMed] [Google Scholar]
- 32.Thumshirn M, Camilleri M, Choi M G.et al Modulation of gastric sensory and motor functions by nitrergic and α2‐adrenergic agents in humans. Gastroenterology 1999116573–585. [DOI] [PubMed] [Google Scholar]
- 33.Rickels K, Pollack M H, Feltner D E.et al Pregabalin for treatment of generalized anxiety disorder. Arch Gen Psychiatry 2005621022–1030. [DOI] [PubMed] [Google Scholar]
- 34.Pande A C, Feltner D E, Jefferson J W.et al Efficacy of the noval anxiolytic pregabalin in social anxiety disorder: a placebo‐controlled, multicenter study. J Clin Psychopharmacol 200424141–149. [DOI] [PubMed] [Google Scholar]
- 35.Pohl R B, Feltner D C, Fieve R R.et al Efficacy of pregabalin in the treatment of generalized anxiety disorder: double‐blind, placebo‐controlled comparison of BID versus TID dosing. J Clin Psychopharmacol 200525151–158. [DOI] [PubMed] [Google Scholar]
- 36.Whitehead W E, Palsson O S. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology 19981151263–1271. [DOI] [PubMed] [Google Scholar]
- 37.Chial H J, Camilleri M, Ferber I.et al Effects of venlafaxine, buspirone and placebo on colonic sensorimotor function in healthy humans. Clin Gastroenterol Hepatol 20031211–218. [DOI] [PubMed] [Google Scholar]
- 38.Jones D L, Sorkin L S. Systemic gabapentin and S(+)‐3‐isobutyl‐γ‐aminobutyric acid block secondary hyperalgesia. Brain Res 199881093–99. [DOI] [PubMed] [Google Scholar]
- 39.Mertz H, Fass R, Kodner A.et al Effect of amitryptiline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol 199893160–165. [DOI] [PubMed] [Google Scholar]
- 40.Tack J, Piessevaux H, Coulie B.et al Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 19981151346–1352. [DOI] [PubMed] [Google Scholar]
- 41.Boeckxstaens G E, Hirsch D P, Kuiken S D.et al The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol 20029740–48. [DOI] [PubMed] [Google Scholar]