Abstract
Objectives
In order to maintain the mucosal barrier against luminal microorganisms the intestinal epithelial cells synthesise various broad‐spectrum antimicrobial peptides including defensins and cathelicidins. Recent studies indicate that both may be deficient in Crohn's disease. To elucidate the possible functional consequences of this deficiency antimicrobial activity in colonic mucosa from patients with inflammatory bowel disease and healthy controls was investigated.
Methods
A flow cytometric assay was established to quantitate bacterial killing and test the antibacterial activity of cationic peptide extracts from colonic biopsies taken from patients with active or inactive ileocolonic or colonic Crohn's disease (n = 22), ulcerative colitis (n = 29) and controls (n = 13) against clinical isolates of Bacteroides vulgatus and Enterococcus faecalis or the reference strains Escherichia coli American Type Culture Collection (ATCC) 25922 and Staphylococcus aureus ATCC 25923.
Results
Compared with controls and ulcerative colitis there was a reduced antimicrobial effect in Crohn's disease extracts that was most evident against B. vulgatus. The antimicrobial effect against E. coli and E. faecalis was significantly lower in Crohn's disease compared with ulcerative colitis. Activity against S. aureus disclosed a similar pattern, but was less pronounced. The differences were independent of the inflammation status or concurrent steroid treatment. Bacteria incubated with biopsy extracts from ulcerative colitis patients frequently showed a characteristic change in cell size and granularity, compatible with more extensive membrane disintegration, compared with bacteria incubated with extracts from controls or Crohn's disease.
Conclusion
Crohn's disease of the colon is characterized by a diminished functional antimicrobial activity that is consistent with the reported low antibacterial peptide expression.
The human intestinal tract hosts more than 500 different microorganism species. With 1012 bacteria per gram of faeces, the colon, in particular, is confronted with the highest bacterial load. Microbial adherence, translocation and infection are prevented by an effective mucosal barrier, including unspecific factors such as luminal bile acids, immunoglobulins and the secretion of mucus. Most importantly, the epithelial lining governs the interaction of the intestinal microorganisms with the host through non‐specific pattern recognition receptors, including the Toll‐like receptors and the NOD receptors, which recognise certain bacterial components.1,2 In addition to the synthesis of cytokines and chemokines, as part of the innate immune system, epithelial cells also produce a variety of cationic antimicrobial peptides to kill bacteria in their immediate vicinity.3 Important representatives of these peptides are the ubiquitous defensins, small cationic peptides with a molecular mass ranging from 3 to 6 kDa and a broad‐spectrum activity against bacteria, fungi and viruses.4 Another relevant family of antimicrobial peptides are the cathelicidins, of which one member (LL‐37) is expressed in the human colon.5,6
Antimicrobial peptide expression in the gastrointestinal tract is either constitutive or inducible. The α‐defensins, human defensin (HD) 5 and HD‐6, which are largely confined to the small intestinal Paneth cells,7 and the human beta defensin (HBD)‐1, which is expressed at multiple epithelial sites including the oesophagus, stomach and colon are expressed constitutively.8,9 In the large intestine, the antibacterial armamentarium is complemented by the major inducible defensins, HBD‐2 and HBD‐3, as well as smaller amounts of HBD‐4, which are expressed in cases of infection or inflammation.10,11,12 This induction is mediated by pro‐inflammatory cytokines such as IL1β mostly through nuclear factor kappa B and activator protein 1‐dependent pathways.13,14 The signalling pathways also include Toll‐like receptors that recognise and bind pathogen‐associated molecular patterns and mitogen‐activated protein kinases.1 In addition, Paneth cell metaplasia at various sites of inflammation along the gastrointestinal tract including the colon provides an alternative “on‐demand” mechanism that enhances antimicrobial expression and protection.15,16
In recent years the important role of the intestinal flora in the pathogenesis of inflammatory bowel disease has received attention.17,18 Swidsinski et al.19 observed high concentrations of adherent and sometimes invading luminal bacteria in inflamed and non‐inflamed colonic biopsies from inflammatory bowel disease. Therefore, the hypothesis was tested that the reduced expression of antimicrobial peptides compromises mucosal host defences and predisposes patients to Crohn's disease of the ileum and colon.20,21 Patients with Crohn's disease of the ileum are characterized by a specific reduction in Paneth cell HD‐5 and HD‐6.22,23 The functional consequence of the low α‐defensin levels was a diminished antibacterial activity in ileal mucosal extracts.23 The relative lack of defensins appears to be associated with E. coli strains adherent to the ileal mucosa.24,25 In contrast, colonic Crohn's disease is characterized by an attenuated induction of inducible β‐defensins, 26,27 partly caused by a reduction in β‐defensin gene copy numbers on chromosome 8.28 Like the defensins, colonic epithelial cathelicidin expression is also heterogeneous, because ulcerative colitis triggers induced expression in contrast to active colonic Crohn's disease,5 possibly further aggravating the barrier defect observed in this disease.
Taken together, the data are compatible with the novel hypothesis that in Crohn's disease altered mucosal antibacterial peptide expression may lead to bacterial overgrowth, epithelial adherence and invasion followed by inflammation.29 In contrast to ileal mucosa,23 in colonic mucosa the functional consequences of diminished defensin and cathelicidin expression remained unproved. We therefore investigated the antimicrobial effect of cationic protein extracts from colonic biopsies taken from patients with Crohn's disease, ulcerative colitis, and controls using an assay recently established for this purpose.30
Materials and methods
Patients and study design
Biopsies taken from the sigmoid colon in 64 patients during routine colonoscopy were frozen immediately in liquid nitrogen. For comparability reasons, sigmoid biopsies were used throughout. The cohort consisted of 13 normal controls, 22 patients with documented colonic Crohn's disease (13 with active macroscopic inflammation in the sigmoid, nine without) and 29 patients with ulcerative colitis (14 inflamed in the sigmoid and 15 non‐inflamed). The study was approved by the ethical committee of the University of Tübingen. All patients gave their written informed consent before colonoscopy.
The demographic data of the patients in this study are given in table 1. In the case of Crohn's disease the disease location was defined by the Vienna classification.31 The inflammatory status was based on macroscopic appearance in the sigmoid where the biopsies were taken (patients may have had more or less active inflammation elsewhere), and was confirmed by histological evaluation and mucosal IL8 messenger RNA expression in tandem biopsies taken from the same site (table 1). Differences in IL8 expression in the respective disease activity groups (non‐inflamed or inflamed) comparing Crohn's disease with ulcerative colitis were not significant (p > 0.05).
Table 1 Demographic data, Vienna classification, steroid treatment and mucosal IL8 expression in controls and patients with Crohn's disease or ulcerative colitis.
| Controls (n = 13) | Crohn's disease (n = 22) | Ulcerative colitis (n = 29) | |||
|---|---|---|---|---|---|
| Non‐inflamed (n = 9) | Inflamed (n = 13) | Non‐inflamed (n = 15) | Inflamed (n = 14) | ||
| Age, years (mean (range)) | 42 (23–78) | 45 (25–69) | 43 (28–83) | 46 (28–66) | 41 (18–78) |
| Sex (% male) | 31% | 44% | 31% | 47% | 29% |
| Vienna classification | – | L2: n = 4 | L2: n = 5 | – | – |
| L3: n = 5 | L3: n = 8 | ||||
| Steroid treatment | – | 44% | 39% | 33% | 50% |
| Mucosal IL8 expression (mRNA copies/10 ng RNA) (SEM) | 19.3 (8.3) | 18.1 (17.3) | 586.9 (390.6) | 89.1 (60.5) | 1325.0 (486.5) |
| p < 0.05 | p < 0.05 | ||||
Extraction of cationic peptides from biopsies
The biopsies were pulverized with a pestle in liquid nitrogen and proteins were extracted under gentle agitation for 2 hours in 5% acetic acid with addition of protease inhibitors (phenylmethylsulfonylfluoride 0.02 mM, pepstatin 2 μg/ml, leupeptin 2 μg/ml). The acid‐soluble proteins in the supernatant were dried under vacuum and resuspended in 0.01% acetic acid. Cationic proteins were extracted overnight at 4°C with a weak cation exchange matrix (Macro Prep CM; Bio‐Rad Laboratories, Hercules, California, USA), added at a 1 : 50 ratio of matrix to extract according to Porter et al.32 with minor modifications. We tested cationic protein extracts because currently known colonic antimicrobial peptides such as defensins, cathelicidins, ubiquicidin, histone 2B, eosinophil cationic protein and phospholipase A2 have cationic properties. After centrifugation, the beads were washed with ammonium acetate followed by elution of the absorbed cations with 5% acetic acid. The cationic eluate was dried under vacuum and resuspended in 0.01% acetic acid. Protein concentrations of the biopsy pellets, the complete supernatants before cationic extraction, the cationic and the non‐cationic fractions were determined by Bradford assay.
Bacterial strains and growth conditions
The bacterial strains used in this study were the clinical strains E. faecalis 199 and B. vulgatus 484 B, both isolated from faeces, and the reference strains E. coli ATCC 25922 and S. aureus ATCC 25923. E. faecalis 199, E. coli ATCC 25922 and S. aureus ATCC 25923 were grown overnight at 37°C in Schaedler broth diluted 1 : 6 with sterile distilled water. Subsequently the bacteria were subcultured in Schaedler broth 1 : 6 and grown to mid‐logarithmic growth phase at 37°C. B. vulgatus 484 B was cultured in Schaedler broth 1 : 6 at 37°C for 2 days in an anaerobic jar.
Antimicrobial assay
The viability of bacteria incubated with biopsy extracts was measured using the membrane potential sensitive dye [bis‐(1,3‐dibutylbarbituric acid) trimethine oxonol] (DiBAC4(3)) (Molecular Probes, Eugene, Oregon, USA) as described previously.30 Bacterial cell wall damage or cell death cause depolarisation of the membrane potential, which results in an uptake of the fluorescent dye.33
Briefly, 1.5×106 mid‐logarithmic‐phase bacteria/ml were incubated in 1 : 6 diluted Schaedler broth at 37°C with cationic biopsy extracts (mean 0.76 μg protein) in a final volume of 100 μl. Each incubation contained the cationic peptides extracted from 15.5 μg total biopsy protein, i.e. the assay was normalised against total biopsy protein to allow for possible variations in cationic protein content between diseases and controls. In fact, the cationic extracts of controls contained an average protein concentration of 0.23 μg/μl, similar to extracts of ulcerative colitis, with 0.29 μg/μl. In extracts of Crohn's disease the average protein concentration was slightly higher, with 0.42 μg/μl.
HBD‐3 (Peptide Institute, Louisville, Kentucky, USA), a defensin with broad‐spectrum antimicrobial activity, served as positive control. HBD‐3 was dissolved in 0.01% acetic acid and added to the bacterial suspensions at a final concentration of 10 μg/ml. Bacterial suspensions incubated with solvent (0.01% acetic acid) instead of biopsy extracts served as controls for the viability of the bacteria.
After 90 minutes the suspensions were incubated for 10 minutes with 1 μg/ml of the dye DiBAC4(3). For time dependence aliquots were taken after 30, 60, 90 and 120 minutes. The suspensions were centrifuged for 10 minutes at 4500g and the bacterial pellets were resuspended in 300 μl phosphate‐buffered saline. The percentage of depolarised fluorescent bacteria in the suspension was determined by flow cytometry.
To confirm these measurements qualitatively we plated out 1 : 10 and 1 : 100 dilutions of bacterial suspensions after 90 minutes incubation with cationic biopsy extracts from three controls, three with Crohn's disease and three with ulcerative colitis on Columbia agar with 5% sheep blood.
Flow cytometry
The viability assay was performed on a FACSCalibur flow cytometer (BD, Sparks, Maryland, USA) as described.30 In each sample 30 000 events were analysed, using Cell Quest software (BD). With the parameters forward scatter and side scatter, referring to relative cell size and granularity, the bacterial population was differentiated from background signals and gated for evaluation of the fluorescence 1. Antimicrobial activity was determined as a percentage of fluorescent bacteria with respect to the untreated control.
Statistical analysis
One‐way analysis of variance was used to compare the percentages of depolarised bacteria in the different disease groups. Tukeys multiple comparison test served as post hoc analysis if analysis of variance revealed significant differences. Probability values of less than 0.05 were considered statistically significant.
Results
Antimicrobial activity of biopsy extracts
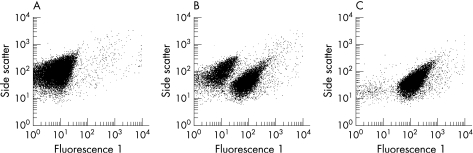
In untreated bacterial suspensions (controls) we observed one dominant population of intact non‐fluorescent bacteria and 1–3% depolarised bacteria, corresponding to the physiological state of bacteria in the mid‐logarithmic growth phase. Cationic protein extracts of biopsies led to a greater proportion of depolarised fluorescent bacteria. Figure 1 illustrates representative fluorescence 1 readings from E. coli ATCC 25922 untreated and incubated with cationic extracts from patients with Crohn's disease and ulcerative colitis.
Figure 1 Representative examples of the fluorescence 1 of E. coli ATCC 25922 after incubation with 0.01% acetic acid as vehicle (A) and biopsy extracts from patients with Crohn's disease (B) and ulcerative colitis (C). After cell damage or cell death the membrane potential sensitive dye [bis‐(1,3‐dibutylbarbituric acid) trimethine oxonol] (DiBAC4(3)) enters the bacterial cells, leading to increased fluorescence 1.
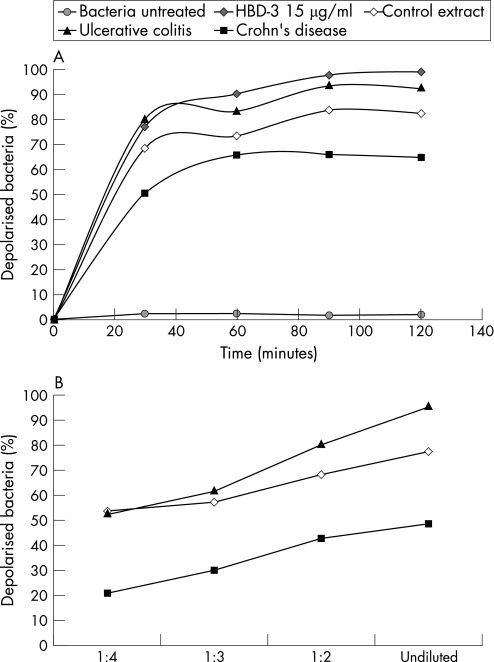
The antimicrobial effect of biopsy extracts was time and protein dependent. After 90 minutes, approximately 80–90% of the bacteria incubated with extracts from a healthy control or a patient with ulcerative colitis were depolarised (fig 2A). Extension of the incubation time up to 120 minutes did not further increase the percentages of bacterial killing. With increasing amounts of cationic proteins the antimicrobial activity rose in parallel (fig 2B).
Figure 2 Time (A) and protein‐dependent (B) antimicrobial activity of representative cationic protein extracts from patients with Crohn's disease, ulcerative colitis and controls against E. coli ATCC 25922.
Different antimicrobial potency of cationic extracts from controls, patients with Crohn's disease and ulcerative colitis
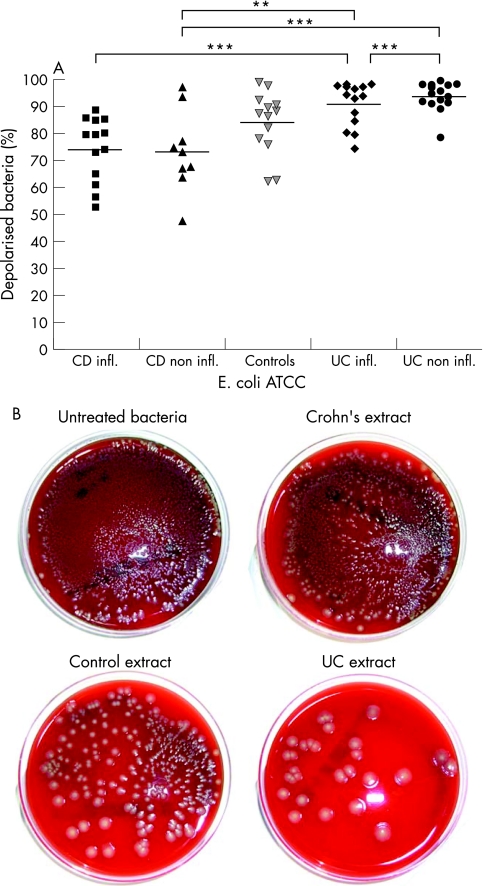
Cationic extracts from sigmoid biopsies exhibited antimicrobial activity against all four bacterial species tested. The strength of the antimicrobial activity in biopsy extracts differed depending on the type of the bacterial species investigated. Antimicrobial activity of extracts from Crohn's disease against E. coli ATCC 25922 was significantly diminished (fig 3). Whereas Crohn's disease extracts from non‐inflamed and inflamed tissue depolarised a mean of 71.5% and 73.6% of E. coli (fig 3A), extracts from ulcerative colitis were more active with 93.9% (non‐inflamed) and 91.2% (inflamed) depolarised bacteria (p < 0.01). These relative potencies of the extracts were qualitatively confirmed by the plating of E. coli ATCC 25922, incubated with cationic extracts of Crohn's disease, controls and ulcerative colitis (fig 3B).
Figure 3 (A) Antimicrobial activity of cationic extracts from biopsies of patients with Crohn's disease (n = 22), ulcerative colitis (n = 29) and controls (n = 13) against E. coli ATCC 25922. ***p < 0.001, **p < 0.01. (B) Representative examples of colony‐forming units of a bacterial suspension of E. coli ATCC 25922, untreated, incubated with cationic extracts of Crohn's disease, of a control or ulcerative colitis.
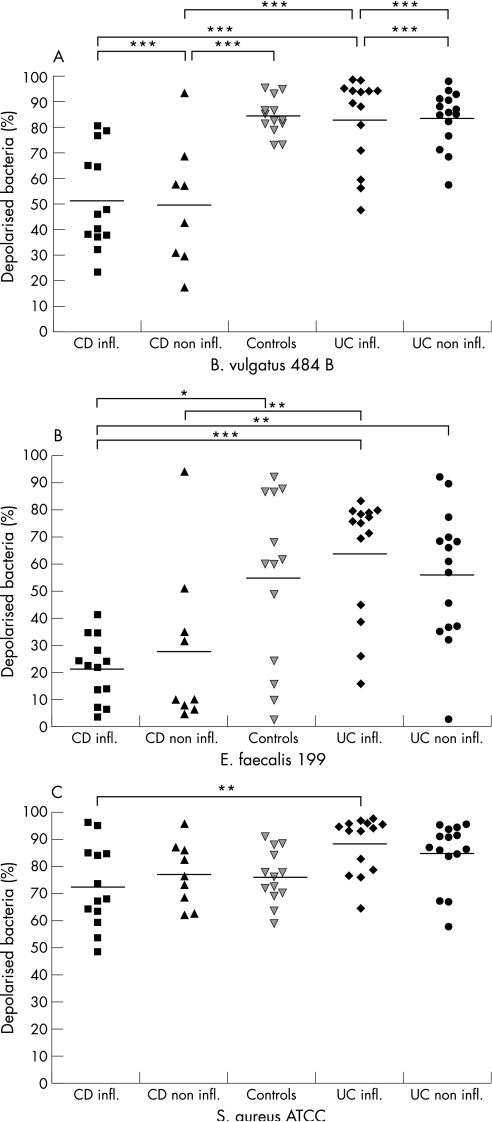
The antimicrobial effect against the anaerobic strain B. vulgatus 484 B (fig 4A) was also significantly lower in extracts from Crohn's disease compared with both ulcerative colitis or controls (p < 0.001). The percentage of depolarised bacteria was comparable in non‐inflamed versus inflamed tissue in Crohn's disease (means 51.5% and 52.8%), but was clearly lower than in non‐inflamed and inflamed ulcerative colitis (81.5% and 80.9%, respectively). Inflammation as such did thus not appear to affect bacterial killing.
Figure 4 Antimicrobial activity of cationic extracts from biopsies of patients with Crohn's disease (n = 22), ulcerative colitis (n = 29) and controls (n = 13) against B. vulgatus 484 B (A), E. faecalis 199 (B) and S. aureus ATCC 25923 (C) ***p < 0.001, **p < 0.01,*p < 0.05.
The antimicrobial activity of biopsy extracts was less pronounced against E. faecalis 199 compared with the other three bacterial strains tested (fig 4B). Incubation with extracts from healthy controls resulted in 54.4% depolarised bacteria. Bacterial killing with extracts from ulcerative colitis amounted to 56.3% in the non‐inflamed state compared with 64.3% in the case of inflammation. The latter was comparable to controls. Extracts of Crohn's disease displayed significantly reduced antimicrobial killing (Crohn's disease versus ulcerative colitis p < 0.01) reaching only 28.1% without and 21.7% with inflammation, respectively.
A similar pattern was observed with S. aureus ATCC 25923, although differences were less pronounced (fig 4C). Depolarised bacteria caused by extracts from Crohn's disease amounted to 75.5% and 72.4% (non‐inflamed and inflamed tissue, respectively) compared with ulcerative colitis, with 84.7% (non‐inflamed) and 88.3% (inflamed).
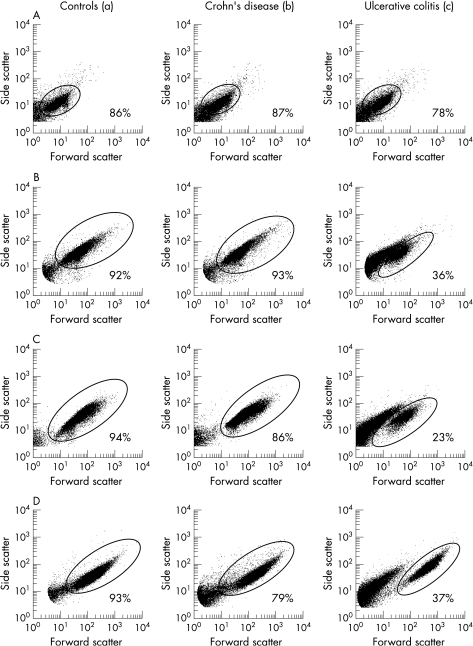
Changes in cell size and granularity of bacteria treated with cationic extracts
E. faecalis 199, E. coli ATCC 25922 and S. aureus ATCC 25923 incubated with biopsy extracts from patients with ulcerative colitis frequently showed a remarkable change in cell size (forward scatter) and granularity (side scatter) as demonstrated in figure 5. This complete disintegration was rarely seen in extracts from controls or in cases of Crohn's disease. This disintegrative effect, which leads to a smaller particle size and lower granularity, was less pronounced with increasing dilution of the cationic extract. Even a concentration of 50 μg/ml HBD‐3 did not lead to a comparable change in bacterial morphology (data not shown). This disintegrative effect was not observed in suspensions of B. vulgatus 484 B treated with cationic extracts.
Figure 5 Forward scatter‐side scatter‐dot plot of B. vulgatus 484 B (A), E. coli ATCC 25922 (B), E. faecalis 199 (C) and S. aureus ATCC 25923 (D) after treatment with cationic extracts from biopsies of controls (a), patients with Crohn's disease (b) and ulcerative colitis (c). After incubation with extracts of ulcerative colitis, in suspensions of E. coli, E. faecalis and S. aureus 50–70% of the bacteria showed a lower forward and side scatter. Bacteria corresponding to the original population are given in percentages of the total.
Discussion
The intestinal bacterial flora plays an important role in the pathogenesis of inflammatory bowel disease.17 With the synthesis of antimicrobial peptides the epithelial cells protect the mucosa from colonisation with bacteria.4,34 A defect in this barrier with diminished synthesis of antimicrobial peptides may lead to bacterial adherence and invasion into the mucosa35,36 and cause inflammation. According to a recent hypothesis,29 this mechanism may represent one important key pathogenic event in Crohn's disease. The present report is in concordance with studies indicating a decreased expression of defensins26,27 as well as cathelicidins5 in the colonic mucosa of Crohn's disease, and is the first to show that endogenous mucosal antimicrobial activity is indeed functionally diminished, particularly in this disease.
In this study antimicrobial activity has been investigated in cationic extracts of both inflamed and uninflamed colonic biopsies of patients with colonic or ileocolonic Crohn's disease and ulcerative colitis. We investigated the Gram‐negative facultative anaerobe species E. coli ATCC 25922 and the anaerobic species B. vulgatus 484 B, representing two prominent species in the intestinal flora. In addition, we tested the Gram‐positive species E. faecalis, which also is an important part of the gut flora,37 and S. aureus ATCC 25923, which was found to be adherent to the mucus.38 To demonstrate a functional deficiency of the antibacterial barrier, we developed a flow cytometric assay,30 which is based on the uptake of dye by depolarised bacteria, measures the bacterial viability at the single cell level, and provides further information about changes in cell morphology. The mechanism of defensins is characterized by an attachment of the cationic peptides to the negatively charged bacterial cell surface, resulting in electrostatic charge‐based membrane permeabilization or in pore formation followed by a membranolytic disruption of the plasma membrane, as shown for HBD‐3.11,39,40 Furthermore, antimicrobial peptides can also inhibit cellular functions in bacteria such as DNA and protein synthesis.41 In the present study the observed depolarisation of bacteria with cationic biopsy extracts was time as well as protein dependent. The percentage of depolarised bacteria reached a plateau between 90 and 120 minutes. A similar time line was observed by Harder et al.11 with HBD‐3. It induced cell wall perforation of S. aureus already at 30 minutes and most of the bacteria had disintegrated after 120 minutes.
In normal uninflamed tissue the wide spectrum of antimicrobial peptides contributing to the epithelial barrier of the colonic mucosa has been investigated by Tollin et al.6 The authors identified a complex mixture of antimicrobial peptides by high‐performance liquid chromatography, including ubiquicidin, histone H2B, phospholipase A2, an eosinophilic cationic protein and the ribosomal protein L39. In addition, LL‐37, the human neutrophil peptides 1–3 and the β‐defensin HBD‐1 were detectable by immunodetection and mass spectrometry. Howell et al.42 found ubiquicidin, histone H2B and histone H1.5 and the ribosomal proteins L30 and S19 in the colonic epithelium. Histones and ubiquicidin are well known as proteins with antimicrobial activity,43,44,45 whereas an antimicrobial role for ribosomal proteins so far remains unclear. Up to now there has been no systematic preparative biochemical study of antimicrobial peptides in the colon of patients with inflammatory bowel disease. With an HBD‐3 antibody we achieved a reduction of 12% in the antimicrobial activity against E. coli ATCC 25922 in a cationic biopsy extract of ulcerative colitis in preliminary experiments (Nuding et al., unpublished data). Unfortunately, the complexity of the antibacterial peptide spectrum and the lack of neutralising antibodies to many known antibacterial peptides precludes the full identification of all mucosal peptides using this approach. Therefore high‐performance liquid chromatography analysis is planned to investigate the antimicrobial peptides involved in detail.
Principally, biopsies from colonic Crohn's disease showed a significant reduction in the antimicrobial activity compared with ulcerative colitis and sometimes with respect to controls. This diminished antimicrobial effect in Crohn's disease may be related to the decreased expression of HBD‐1 and diminished induction of HBD‐2, HBD‐3 and HBD‐4 in the mucosa.26,27,29 On the other hand, the greater activity in ulcerative colitis compared with Crohn's disease is in accordance with an increased expression of these β‐defensins and a significant increase in the expression of the constitutive cathelicidin LL‐37 in inflamed as well as in non‐inflamed colonic biopsies of patients with ulcerative colitis, as described recently.5
The antimicrobial activity was diminished in Crohn's disease, although the average amount of cationic proteins in biopsy extracts of Crohn's disease patients, when normalised to total biopsy protein, was even higher than in ulcerative colitis and controls. This leads to the conclusion that important antimicrobial peptides such as defensins and cathelicidins appear to be expressed at lower levels or to be defective, whereas other cationic proteins with or without minor antimicrobial function may be augmented. Also, it should be noted that this functional assay did not simply reflect β‐defensin expression, which in the absence of inflammation is consistently lower in both diseases than in its presence.27 Interestingly, inflamed compared with non‐inflamed tissues had a similar antibacterial functional activity in both diseases, suggesting that subepithelial neutrophil‐derived peptides played a minor role. To quantitate more directly a potential contribution of inflammatory cells to biopsy antibacterial activity, we measured bacterial killing by extracts from lymphocytes or granulocytes. The data imply only a minor antibacterial effect with 17–26% killing (unpublished data). Consistent with a major contribution of epithelial antibacterial peptides, extensive staining of various antibacterial peptides including HBD‐1, HBD‐2,46 cathelicidine5 and elafin47 in the epithelium also suggests that this is the major cellular source.
Preliminary investigations regarding the antimicrobial spectrum of defensins against anaerobic bacterial strains showed that the most effective defensin against B. vulgatus and B. fragilis is HBD‐3 (Nuding et al., unpublished data). In concentrations that kill E. coli and S. aureus, the constitutive HBD‐1 exhibits in vitro no antimicrobial activity against B. vulgatus. Therefore, the described decreased induction of HBD‐326,27 and the deficient activity of HBD‐1 may be responsible for a lower antimicrobial activity in Crohn's disease against B. vulgatus. The cationic LL‐37 also displays broad‐spectrum antimicrobial activity,46 and the absence of induction in Crohn's disease5 may add to the failure of the antimicrobial agents. Furthermore, inflammatory bowel diseases are associated with an increased expression of α‐defensins and lysozyme in colonic epithelium,15,48 attributable to colonic Paneth‐cell metaplasia.
Using the flow cytometric technique, we could also detect a particularly pronounced qualitative change in the cell morphology of E. coli ATCC 25922, E. faecalis 199 and S. aureus ATCC 25923 following incubation with protein extracts of biopsies taken from patients with ulcerative colitis. Only a near complete disintegration of the cells could cause the observed decreased bacterial cell size and diminished granularity. This change in cell morphology after incubation with extracts from ulcerative colitis biopsies may be caused by higher levels of antimicrobial peptides, including a possible synergistic effect. Comparable changes were recently described for Bacillus subtilus treated with Nisin, a small cationic lanthionine antibiotic.49
The antimicrobial activity of extracts from ulcerative colitis, with a tendency to increased activity compared with controls, clearly demonstrates that the pathogenic defect in ulcerative colitis is not mediated by the diminished synthesis of antimicrobial peptides as in Crohn's disease. In contrast to the almost sterile mucus of healthy controls, however, colonisation of the mucus with microorganisms is characteristic for both Crohn's disease and ulcerative colitis.50 At the epithelium, a recent study failed to find adherent bacteria in ulcerative colitis,36 which is consistent with our findings. In that disease there is possibly a different problem in maintaining the antibacterial mucus barrier that is the focus of current work.
It seems likely that these host factors impact on the bacterial flora under normal and inflammatory conditions. The dysbiosis described in inflammatory bowel diseases51,52 may thus be caused by these alterations of mucosal bacterial killing. For example, there is an increased prevalence of mucosa‐associated E. coli in Crohn's disease compared with ulcerative colitis or controls.19,36,53,54 Data regarding the prevalence of Bacteroides species are inconsistent. Several studies have reported an increased colonization of the mucosa with Bacteroides species19,55,56 in inflammatory bowel disease, whereas other data show unchanged or lower levels of Bacteroides.54,57 In conclusion, the present finding in Crohn's disease of a compromised functional antibacterial activity compared with ulcerative colitis, and in some bacterial species also against controls, may represent an important and likely primary pathogenic mucosal defect of colonic Crohn's disease.
Acknowledgements
This study was supported by the Robert Bosch Foundation, Stuttgart, Germany. In addition, Jan Wehkamp is an Emmy Noether Scholar of the Deutsche Forschungsgemeinschaft. The authors are grateful to Dagmar Weller for excellent technical assistance. They also appreciate the support of Holger A G Mueller in performing some experiments in the Institute for Laboratory Medicine of the Klinik am Eichert Göppingen, and also thank Lutz Zabel for helpful discussion and support, especially his advice on working with anaerobic bacteria.
Abbreviations
ATCC - American Type Culture Collection
DiBAC4(3) - [bis‐(1,3‐dibutylbarbituric acid) trimethine oxonol]
HBD - human β‐defensin
HD - human defensin
Footnotes
Conflict of interest: None declared.
References
- 1.Abreu M T, Fukata M, Arditi M. TLR Signaling in the Gut in Health and Disease. J Immunol 20051744453–4460. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Murray P J, Kitani A.et al Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 200669–20. [DOI] [PubMed] [Google Scholar]
- 3.Lehrer R I, Bevins C L. Defensins and other antimicrobial peptides. In: Mestecky J, Bienenstock J, Lammet ME, et al., eds. Mucosal immunology. New York: Academic Press, 200495–110.
- 4.Bevins C L, Martin‐Porter E, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut 199945911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauber J, Rieger D, Weiler F.et al Heterogeneous expression of human cathelicidin hCAP18/LL‐37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol 200618615–621. [DOI] [PubMed] [Google Scholar]
- 6.Tollin M, Bergman P, Svenberg T.et al Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 200324523–530. [DOI] [PubMed] [Google Scholar]
- 7.Bevins C L. The Paneth cell and the innate immune response. Curr Opin Gastroenterol 200420572–580. [DOI] [PubMed] [Google Scholar]
- 8.Eckmann L. Defence molecules in intestinal innate immunity against bacterial infections. Curr Opin Gastroenterol 200521147–151. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C, Wang I, Lehrer R I. Widespread expression of beta‐defensin hBD‐1 in human secretory glands and epithelial cells. FEBS Lett 1996396319–322. [DOI] [PubMed] [Google Scholar]
- 10.Garcia J R C, Krause A, Schulz S.et al Human {beta}‐defensin 4: a novel inducible peptide with a specific salt‐sensitive spectrum of antimicrobial activity. FASEB J 2001151819–1821. [PubMed] [Google Scholar]
- 11.Harder J, Bartels J, Christophers E.et al Isolation and characterization of human beta‐defensin‐3, a novel human inducible peptide antibiotic. J Biol Chem 20012765707–5713. [DOI] [PubMed] [Google Scholar]
- 12.Harder J, Bartels J, Christophers E.et al A peptide antibiotic from human skin. Nature 1997387861. [DOI] [PubMed] [Google Scholar]
- 13.O'Neil D A, Porter E M, Elewaut D.et al Expression and regulation of the human beta‐defensins hBD‐1 and hBD‐2 in intestinal epithelium. J Immunol 19991636718–6724. [PubMed] [Google Scholar]
- 14.Wehkamp J, Harder J, Wehkamp K.et al NF‐{kappa}B‐ and AP‐1‐mediated induction of human beta defensin‐2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 2004725750–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunliffe R N, Rose F R A J, Keyte J.et al Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 200148176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen B, Porter E M, Reynoso E.et al Human defensin 5 expression in intestinal metaplasia of the upper gastrointestinal tract. J Clin Pathol 200558687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartor R B. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 20063390–407. [DOI] [PubMed] [Google Scholar]
- 18.Farrell R J, LaMont J T. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am 20023141–62. [DOI] [PubMed] [Google Scholar]
- 19.Swidsinski A, Ladhoff A, Pernthaler A.et al Mucosal flora in inflammatory bowel disease. Gastroenterology 200212244–54. [DOI] [PubMed] [Google Scholar]
- 20.Wehkamp J, Fellermann K, Herrlinger K R.et al Mechanisms of disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol 20052406–415. [DOI] [PubMed] [Google Scholar]
- 21.Wehkamp J, Schmid M, Fellermann K.et al Defensin deficiency, intestinal microbes, and the clinical phenotypes of Crohn's disease. J Leukoc Biol 200577460–465. [DOI] [PubMed] [Google Scholar]
- 22.Wehkamp J, Harder J, Weichenthal M.et al NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha‐defensin expression. Gut 2004531658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehkamp J, Salzman N H, Porter E.et al Reduced Paneth cell alpha‐defensins in ileal Crohn's disease. Proc Natl Acad Sci U S A 200510218129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darfeuille‐Michaud A, Neut C, Barnich N.et al Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 19981151405–1413. [DOI] [PubMed] [Google Scholar]
- 25.Darfeuille‐Michaud A, Boudeau J, Bulois P.et al High prevalence of adherent‐invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 2004127412–421. [DOI] [PubMed] [Google Scholar]
- 26.Fahlgren A, Hammarstrom S, Danielsson A.et al Beta‐defensin‐3 and ‐4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin Exp Immunol 2004137379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehkamp J, Harder J, Weichenthal M.et al Inducible and constitutive beta‐defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 20039215–223. [DOI] [PubMed] [Google Scholar]
- 28.Fellermann K, Stange D E, Schaeffeler E.et al A chromosome 8 gene‐cluster polymorphism with low human beta‐defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet 200679439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellermann K, Wehkamp J, Herrlinger K R.et al Crohn's disease: a defensin deficiency syndrome? Eur J Gastroenterol Hepatol 200315627–634. [DOI] [PubMed] [Google Scholar]
- 30.Nuding S, Fellermann K, Wehkamp J.et al A flow cytometric assay to monitor antimicrobial activity of defensins and cationic tissue extracts. J Microbiol Methods 200665335–345. [DOI] [PubMed] [Google Scholar]
- 31.Gasche C, Scholmerich J, Brynskov J.et al A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 200068–15. [DOI] [PubMed] [Google Scholar]
- 32.Porter E M, Poles M A, Lee J S.et al Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett 1998434272–276. [DOI] [PubMed] [Google Scholar]
- 33.Jepras R I, Paul F E, Pearson S C.et al Rapid assessment of antibiotic effects on Escherichia coli by bis‐(1,3‐ dibutylbarbituric acid) trimethine oxonol and flow cytometry. Antimicrob Agents Chemother 1997412001–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunliffe R N, Mahida Y R. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol 20047549–58. [DOI] [PubMed] [Google Scholar]
- 35.Swidsinski A, Weber J, Loening‐Baucke V.et al Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 2005433380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin H M, Campbell B J, Hart C A.et al Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 200412780–93. [DOI] [PubMed] [Google Scholar]
- 37.Conte M P, Schippa S, Zamboni I.et al Gut‐associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006551760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesterlund S, Karp M, Salminen S.et alStaphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology 20061521819–1826. [DOI] [PubMed] [Google Scholar]
- 39.Kagan B L, Selsted M E, Ganz T.et al Antimicrobial defensin peptides form voltage‐dependent ion‐permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A 199087210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimoda M, Ohki K, Shimamoto Y.et al Morphology of defensin‐treated Staphylococcus aureus. Infect Immun 1995632886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brogden K A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 20053238–250. [DOI] [PubMed] [Google Scholar]
- 42.Howell S J, Wilk D, Yadav S P.et al Antimicrobial polypeptides of the human colonic epithelium. Peptides 2003241763–1770. [DOI] [PubMed] [Google Scholar]
- 43.Hiemstra P S, van den Barselaar M T, Roest M.et al Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J Leukoc Biol 199966423–428. [DOI] [PubMed] [Google Scholar]
- 44.Parseghian M H, Luhrs K A. Beyond the walls of the nucleus: the role of histones in cellular signaling and innate immunity. Biochem Cell Biol 200684589–604. [DOI] [PubMed] [Google Scholar]
- 45.Rose F R, Bailey K, Keyte J W.et al Potential role of epithelial cell‐derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect Immun 1998663255–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehkamp J, Fellermann K, Herrlinger K R.et al Human beta‐defensin 2 but not beta‐defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol 200214745–752. [DOI] [PubMed] [Google Scholar]
- 47.Schmid M, Fellermann K, Fritz P.et al Attenuated induction of epithelial and leukocyte serine antiproteases elafin and secretory leukocyte protease inhibitor in Crohn's disease. J Leukoc Biol 200781907–915. [DOI] [PubMed] [Google Scholar]
- 48.Wehkamp J, Schwind B, Herrlinger K R.et al Innate immunity and colonic inflammation: enhanced expression of epithelial alpha‐defensins. Dig Dis Sci 2002471349–1355. [DOI] [PubMed] [Google Scholar]
- 49.Hyde A J, Parisot J, McNichol A.et al Nisin‐induced changes in Bacillus morphology suggest a paradigm of antibiotic action. Proc Natl Acad Sci U S A 200610319896–19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultsz C, Van Den Berg F M, Ten Kate F W.et al The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology 19991171089–1097. [DOI] [PubMed] [Google Scholar]
- 51.Ott S J, Musfeldt M, Wenderoth D F.et al Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 200453685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seksik P, Sokol H, Lepage P.et al Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther 200624(Suppl 3)11–18. [DOI] [PubMed] [Google Scholar]
- 53.Kleessen B, Kroesen A J, Buhr H J.et al Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol 2002371034–1041. [DOI] [PubMed] [Google Scholar]
- 54.Mylonaki M, Rayment N B, Rampton D S.et al Molecular characterization of rectal mucosa‐associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis 200511481–487. [DOI] [PubMed] [Google Scholar]
- 55.Bibiloni R, Mangold M, Madsen K L.et al The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn's disease and ulcerative colitis patients. J Med Microbiol 2006551141–1149. [DOI] [PubMed] [Google Scholar]
- 56.Ruseler‐van Embden J G, Both‐Patoir H C. Anaerobic gram‐negative faecal flora in patients with Crohn's disease and healthy subjects. Antonie Van Leeuwenhoek 198349125–132. [DOI] [PubMed] [Google Scholar]
- 57.Seksik P, Rigottier‐Gois L, Gramet G.et al Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 200352237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]