Abstract
Background
In patients with non‐erosive gastroesophageal reflux disease, heartburn can occur when acid reaches sensory nerve endings through oesophageal‐mucosa‐dilated intercellular spaces. Stressful life events may increase heartburn perception. In the rat, acute stress increases gastric and intestinal mucosa permeability. We investigated whether acute stress can also increase oesophageal mucosa permeability and contribute to the dilation of mucosa intercellular spaces.
Methods
Male Sprague–Dawley rats were submitted to partial restraint stress. Oesophageal mucosa from stressed and control rats was mounted in diffusion chambers. The permeability to 51Cr‐EDTA (400 Da), fluorescein isothiocyanate (FITC)‐dextran 4000 Da (FD4) and FITC‐dextran 20 000 Da (FD20) was assessed after tissue incubation either with Krebs (control) or HCl pH 2.0+ pepsin 1 mg/ml. The diameter of intercellular spaces was assessed using transmission electron microscopy.
Results
Acute stress increased faecal output, small‐intestinal permeability and glycaemia. Exposure of oesophageal mucosa from control rats to acid‐pepsin did not increase permeability to any of the tested molecules. Stress increased the number of submucosal mast cells and, by itself, increased the permeability to the smallest molecule (22.8±7.1 pmol/cm2 vs 5.8±2.1 pmol/cm2) (p<0.001). Exposure of mucosa from stressed rats to acid‐pepsin significantly increased permeability to all molecules tested. Electron microscopy showed dilated intercellular spaces only in mucosa from stressed rats (with and without exposure to acid‐pepsin).
Conclusions
Acute stress can increase, by itself, oesophageal mucosa permeability. There is a potentiation between stress and exposure of the oesophageal mucosa to acid‐pepsin, leading to increased permeability and dilated intercellular spaces.
Keywords: stress, permeability, dilated intercellular spaces, rat, oesophageal mucosa
In patients with non‐erosive gastroesophageal reflux disease (GERD), heartburn is thought to occur when acid and/or other components of the refluxed gastric content reach sensory nerve endings through oesophageal‐mucosal‐dilated intercellular spaces (DIS).1,2,3 It has been proposed that dilation of the intercellular spaces results in an increase in paracellular permeability, thereby facilitating acid to reach chemoreceptors that are located in the mucosa.2,4 Intra‐epithelial nerve endings of spinal afferents are likely to be involved in the mediation of acid‐induced oesophageal symptoms.5 Previous experimental studies have suggested that DIS is a secondary phenomenon induced by mucosal exposure to acid, pepsin and bile acids,3,6 and a recent study in humans showed that DIS can be reversible after adequate control of oesophageal acid exposure with proton pump inhibitors.7
Several factors can determine the characteristics and intensity of reflux‐induced oesophageal symptoms. They include acidity, volume and proximal extent of reflux; presence of Barrett's epithelium; age; and presence of lipids in the duodenum.8 Additionally, central mechanisms can modulate the perception of intra‐oesophageal stimuli, through brain–gut interactions. For example, stress or anxiety may increase heartburn perception.9,10 Central hypersensitivity is currently the most accepted hypothesis for stress‐increased oesophageal symptoms.11,12
Stress can increase the permeability of both simple columnar epithelium in the gastrointestinal tract and stratified squamous epithelium in the skin. In the stomach, experimental acute stress increases mucosal permeability13,14 and, together with acid, plays an important role in the pathogenesis of gastric ulcerations.15,16 Similarly, in the small bowel and colon, stress increases mucosa paracellular transport.17,18,19 In the skin, stress can alter cutaneous permeability by decreasing corneodesmosomes.20
We hypothesized that acute stress could also affect the oesophageal epithelium, increasing paracellular permeability and contributing to the dilation of intercellular spaces. Thereby, stress could be involved in the pathophysiology of reflux‐induced oesophageal symptoms.
The aim of this study was to evaluate the effect of experimental acute stress on oesophageal mucosa permeability and intercellular spaces in rats.
Materials and Methods
Animals
The experiments were performed in adult male Sprague–Dawley rats weighing approximately 150–180 g. The animals were kept in individual plastic cages, in a temperature‐controlled environment (20–22 °C) under a 13/11 h light/dark cycle, and provided with food and water ad libitum. In order to reduce manipulation‐induced stress, rats were handled daily by the same investigator for 1 week before the study. The experiments were approved by the ethical committee for animal experiments of the Catholic University of Leuven, Belgium.
Stress model
The experiments were always performed at room temperature between 10:00 and 13:00 h. After 1 week of daily manipulation, rats were divided into two groups: 1: control rats were maintained in their home cage for 4 h; 2: stressed rats underwent Partial Restraint Stress (PRS), a previously described experimental model of acute stress used in visceral hypersensitivity and intestinal permeability studies.21,22 This model is considered to be of mild intensity; PRS is a non‐ulcerogenic stressor22 that activates the hypothalamic‐pituitary‐adrenal (HPA) axis23 and involves elements of physical stress in addition to psychological stress.24 The animal's fore‐shoulders, upper fore‐limbs and thoracic trunk were wrapped in a cloth harness to restrict, but not prevent, body movements for 2 h. This period was followed by 2 h of free movement in the home cage. The time schedule was selected because previous studies reported a maximal increase in intestinal permeability 2 h after the restraint stress.19 Faecal pellet output and glycaemia are known to increase under stress conditions.25,26 Faeces were collected and blood samples for glycaemia determination were obtained by tail nicks during the experiments. These parameters were used to assess the effectiveness of the model for stress induction.
After completing the protocol, all rats were sacrificed by stunning and posterior exsanguinations.
Permeability studies
The complete oesophagus was excised, opened and stripped of its muscle layers in a paraffin tray containing carbogenated Krebs–Henseleit bicarbonate buffer (KHBB; pH 7.4, containing (in mM): 118 NaCl; 4.7 KCl; 1.2 CaCl2; 1.2 MgSO4; 1.2 NaH2PO4; 25 NaHCO3; and 11 glucose) so that a sheet of mucosal tissue was obtained, consisting of stratified squamous epithelium and underlying connective tissue. A similar procedure was performed with small intestine obtained from the same animal.
Oesophageal mucosal sections of approximately 0.5 cm2 (n = 4) were cut and mounted in a diffusion chamber for measurements of permeability to small (400 Da), medium (4000 Da) and large (20 000 Da) molecules using 51Cr‐EDTA (Amersham International, Amersham, UK), fluorescein isothiocyanate (FITC)‐dextran 4 (FD4) and FITC‐dextran 20 (FD20) (Sigma/RBI, Belgium), respectively.
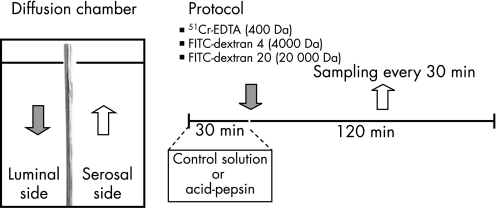
The diffusion chamber allowed for exposure of the luminal side of the tissue to different test solutions and regular sampling from the serosal side to detect the degree of mucosal permeability to different molecules (fig 1).
Figure 1 Schematic representation of permeability studies and protocol. Oesophageal mucosal sections of approximately 0.5 cm2 were cut and mounted in a diffusion chamber. The diffusion chamber allowed for exposure of the luminal side of the tissue to different test solutions and regular sampling from the serosal side to detect the degree of mucosal permeability to different molecules.
Tissues were bathed in 37 °C carbogenated KHBB (3.2 ml on each side) for 40 min. Then, the luminal side was exposed for 30 min to either a control solution (KHBB pH 7.4) or to a solution containing acid‐pepsin (HCl pH 2.0 plus porcine pepsin A 1 mg/ml) (392 units/mg solid). A similar protocol was previously used by Tobey et al. to provoke DIS and increased paracellular permeability in rabbit oesophageal mucosa.3
After this period, the solutions in the luminal side were replaced by solution containing either 51Cr‐EDTA (6 μCi/ml), FITC‐dextran 4 (1 mg/ml) or FITC‐dextran 20 (1 mg/ml). A 300‐μl sample was taken from the luminal side to determine the initial concentration. Samples (300 μl) from the serosal side of the diffusion chamber were obtained at 0, 30, 60, 90 and 120 min. Volume in both sides of the diffusion chambers was kept constant by adding normal KHBB. The permeability to molecules of increasing molecular weight was measured as follows: a β liquid scintillation counter (Packard, model 2100, Downers Grove, IL) was used to detect 51Cr‐EDTA. Luminal‐to‐serosal fluxes of 51Cr‐EDTA were calculated and expressed as nmol cm–2. A fluorescence‐plate reader (Fluoroskan, Ascent, Thermo LabSystems, Belgium) was used to detect FITC‐dextran. The fluorescence of the supernatant was measured using an excitation wavelength of 485 nm and an emission wavelength of 538 nm. Luminal‐to‐serosal fluxes of FITC‐dextran were calculated and expressed as pmol cm–2. In addition, luminal‐to‐serosal flux was expressed as the slope of the concentration/surface/time curves for each experimental condition.
Morphological studies
Following the permeability experiments in diffusion chambers, tissues were examined using both light and transmission electron microscopy (TEM). Tissues were fixed in 4% (w/v) paraformaldehyde for light microscopy and in 2.5% (w/v) glutaraldehyde in phosphate buffer for TEM. Light microscopy was performed embedding the tissue in paraffin. Transverse sections (5 μm) were stained using haematoxylin‐eosin and von Gieson methods. Toluidine blue staining was performed to quantify mast cells. The sections were stained with acidified (pH 2.5) toluidine blue (Sigma, St. Louis) and mast cells were counted at ×400 magnification in 60 fields.
For TEM, tissues were post‐fixed in 1% buffered osmium tetroxide at 4 °C, and dehydrated through a graded alcohol series, then embedded in an epoxy resin. Ultrathin sections were post‐stained with uranyl acetate lead citrate. Specimens were examined and photographed using a Zeiss transmission electron microscope. Two TEM photos/per animal were taken (×4000 magnification) and analysed using custom‐written image analysis software in IGOR Pro (WaveMetrics Inc., Oregon, USA). Intercellular spaces were delineated between 5–10 epithelial cells from the basal layer in each microphotograph. The intercellular space area was measured and compared with the perimeter of the corresponding cells to obtain a relative measure of DIS.27
The morphological evaluations were performed blinded to the type of mucosal exposure and results of the permeability studies.
Statistics
All data is expressed as mean ± SEM. Single comparisons were performed by paired or unpaired Student's t‐test when appropriate. The effect of stress and acid‐pepsin on time‐permeability curves was analysed using two‐way repeated measures ANOVA. When the ANOVA test was significant, the Bonferroni test was used to determine the times with statistical significant difference. Significance was declared at p<0.05.
Results
Effectiveness of the partial restraint model for stress induction
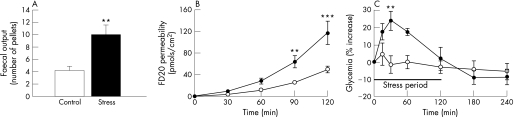
A 2‐hour period of partial restraint induced significant changes in different parameters that are typically affected by stress. Intestinal propulsive motor activity was increased, provoking a significantly higher faecal pellet output in stressed rats compared with controls (9.9 ± 1.4 pellets vs 4.4 ± 0.6; p<0.01) (fig 2A). Intestinal mucosa from stressed rats was significantly more permeable than mucosa from control rats, as indicated by increased luminal‐to‐serosal flux of FD20 (p<0.0001) at 90 and 120 min (64.0 ± 11.0 vs 25.6 ± 2.7 pmols/cm2 and 117.2 ± 21.4 vs 49.8 ± 6.0 pmols/cm2, respectively) (fig 2B). Glycaemia was significantly increased in stressed rats compared with controls at 30 min of the immobilization period (24.0 ± 5.4%; p<0.01) (fig 2C).
Figure 2 Effect of acute stress on faecal output, intestinal permeability and glycaemia. Stressed rats (filled symbols) and control rats (open symbols). (A) Stress increased faecal pellet expulsion. (B) Stress increased intestinal permeability to FITC‐dextran 20 at 90 and 120 min. (C) Stress increased glycaemia (expressed as percentage change after immobilization). Data expressed as mean ± SEM; stress vs control: **p<0.01 and ***p<0.001
Effect of acid‐pepsin exposure on oesophageal mucosa permeability
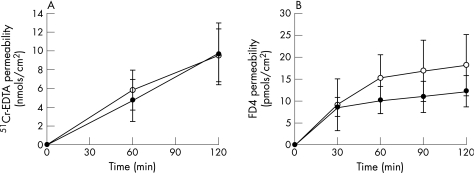
Exposure of the oesophageal mucosa of control rats to acid‐pepsin solutions did not increase oesophageal mucosa permeability to the tested molecules. This was the case not only for medium‐sized molecules (4000 Da) but also for the smallest molecule tested (400 Da). After 120 min, the permeability for 51Cr‐EDTA (400 Da) was 9.5 ± 2.8 nmols/cm2 and 9.7 ± 3.2 nmols/cm2 following exposure to control solution (KHBB) and acid‐pepsin solution, respectively (fig 3A). The flux (slope) was not significantly different between both conditions (0.08 ± 0.01 vs 0.08 ± 0.01 nmols/cm2/min; p = 0.68). Similarly, there was no significant difference in permeability to FD4 (4000 Da) following mucosal exposure to control solution (KHBB) (18.2 ± 9.0 pmols/cm2) or acid‐pepsin solution (12.3 ± 3.5 pmols/cm2) (fig 3B) (p = ns). The flux was not significantly different between both conditions (0.18 ± 0.12 vs 0.14 ± 0.02 nmols/cm2/min; p = 0.16).
Figure 3 Effect of acid‐pepsin exposure on oesophageal mucosa permeability. Acid‐pepsin solution (filled symbols) and control solution (open symbols). Exposure of the oesophageal mucosa of control rats to acid‐pepsin solutions did not increase oesophageal mucosa permeability to the tested molecules: (A) 51Cr‐EDTA (400 Da) and (B) FD4 (4000 Da). Data expressed as mean ± SEM; n = 5 rats/group, with two tissues averaged per rat.
Effect of stress on oesophageal mucosa permeability
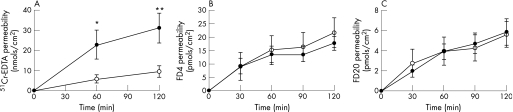
Acute stress, by itself, increased oesophageal mucosa permeability. The permeability for 51Cr‐EDTA (400 Da) was significantly higher in oesophageal mucosa from stressed rats compared with tissue from control rats, both at 60 (22.8 ± 7.1 pmol/cm2 vs 5.8 ± 2.1 pmol/cm2; p<0.001) and 120 min (31.1 ± 7.4 pmol/cm2 vs 9.5 ± 2.8 pmol/cm2; p<0.001) (fig 4A). Flux was significantly higher in stressed animals (0.29 ± 0.04 vs 0.08 ± 0.02 nmols/cm2/min; p<0.0001). This effect was only observed with the smallest molecule. Stress did not increase mucosal permeability for medium size (4000 Da) (21.7 ± 5.5 pmols/cm2 vs 17.7 ± 2.6 pmols/cm2; flux 0.19 ± 0.01 vs 0.22 ± 0.03 nmols/cm2/min) (fig 4B) and larger molecules (20000 Da) (5.6 ± 1.3 vs 5.9 ± 1.3 pmols/cm2; flux 0.06 ± 0.005 vs 0.06 ± 0.008 nmols/cm2/min) (fig 4C). The changes provoked by stress in the oesophagus were substantially less marked than those observed in the intestine (intestinal permeability for FD20 (20 000 Da) at 120 min was 117.2 ± 21.4 pmol/cm2).
Figure 4 Effect of stress on oesophageal mucosa permeability. Acute stress, by itself, increased oesophageal mucosa permeability. Stressed rats (filled symbols) and control rats (open symbols). (A) The permeability for 51Cr‐EDTA (400 Da) was significantly higher in oesophageal mucosa from stressed rats compared with tissue from control rats, both at 60 and 120 min. (B) Stress did not increase mucosal permeability to medium FD4 (4000 Da) and (C) larger molecules FD20 (20 000 Da). Data expressed as mean ± SEM; n = 5–8 rats/group, with two tissues averaged per rat; stress vs control: *p<0.05 and **p<0.01.
Combined stress and acid‐pepsin exposure on oesophageal mucosa permeability
The combination of stress with acid‐pepsin exposure provoked the most significant increase in oesophageal mucosa permeability.
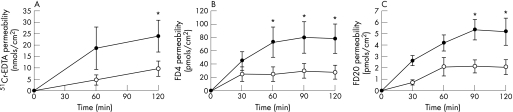
Exposure of mucosa from stressed rats to a solution with acid‐pepsin increased the permeability for 51Cr‐EDTA (p = 0.016) compared with controls (non‐stressed rats). At 120 min, oesophageal permeability to 51Cr‐EDTA was 23.8.0 ± 7.1 pmol/cm2 compared with 9.7 ± 3.2 pmol/cm2 in controls and the flux was 0.19 ± 0.04 vs 0.08 ± 0.01 nmols/cm2/min, respectively (p<0.0001) (fig 5A). This increase was not higher than that observed after stress alone. Furthermore, unlike stress alone or acid‐pepsin alone, exposing the mucosa from stressed rats to acid‐pepsin significantly increased the permeability to medium‐sized molecules. The permeability to FD4 (4000 Da) was significantly enhanced at 60, 90 and 120 min (72.8 ± 22.4 vs 24.4 ± 10.6 pmols/cm2, 79.9 ± 23.2 vs 29.5 ± 11.0 pmols/cm2 and 78.1 ± 21.9 vs 29.8 ± 10.2 pmols/cm2, respectively) and the flux was 1.06 ± 0.14 vs 0.41 ± 0.07, respectively (p<0.0001) (fig 5B). Although the permeability to FD20 (20000 Da) was significantly higher at 90 and 120 min (5.3 ± 0.9 vs 2.1 ± 0.6 pmols/cm2 and 5.2 ± 1.2 vs 2.1 ± 0.7, respectively), this difference was due to a slight decrease in permeability after acid‐pepsin alone (fig 5C).
Figure 5 Combined stress and acid‐pepsin exposure on oesophageal mucosa permeability. Stressed rats (filled symbols) and control rats (open symbols). The combination of stress with acid‐pepsin exposure provoked the most significant increase in oesophageal mucosa permeability. (A) Exposure of mucosa from stressed rats to a solution with acid‐pepsin increased the permeability for 51Cr‐EDTA. (B) The permeability to FD4 (4000 Da) was significantly enhanced at 60, 90 and 120 min. (C) The permeability to FD20 (20 000 Da) was significantly increased at 90 and 120 min. Data is expressed as mean ± SEM; n = 5–10 rats/group, with two tissues averaged per rat; stress vs control: *p<0.05.
Morphological studies
Light microscopy showed no evidence of erosions or cell necrosis in mucosa exposed to acid‐pepsin from control or stressed rats. No typical features of human oesophagitis were observed (inflammatory infiltration, papillae elongation or hypertrophy of basal layer).
The number of submucosal mast cells was significantly higher in stressed rats compared with controls. Toluidine blue staining showed 244 ± 6 mast cells/60 fields in stressed rats and 193 ± 15 mast cells/60 fields in control rats (p<0.05).
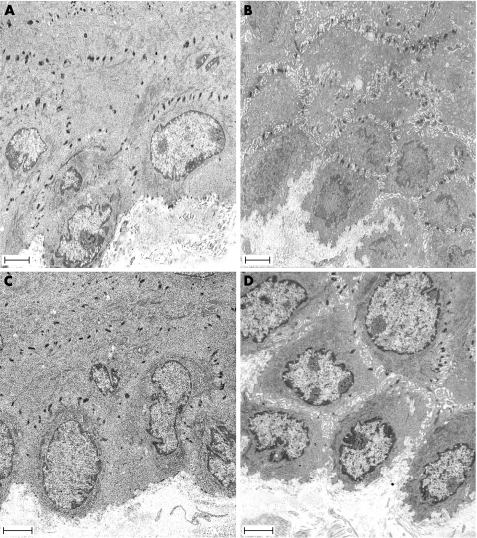
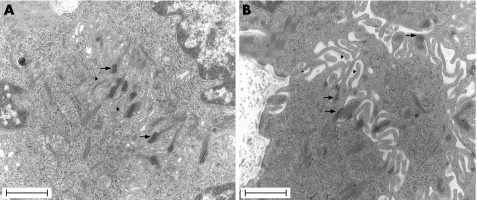
The effect of stress on oesophageal mucosa permeability was clearly observed with TEM. Exposure of the oesophageal mucosa to acid‐pepsin solutions did not significantly increase the relative area of intercellular spaces compared with controls. In contrast, stress by itself significantly increased the relative area of intercellular spaces (0.19 ± 0.04 μm vs 0.10 ± 0.02; n = 4; p<0.05) (fig 6B). Furthermore, the combination of stress with acid‐pepsin exposure (fig 6D) was associated with larger relative areas of intercellular spaces than that observed after mucosal exposure to acid‐pepsin alone (fig 6C) (0.11 ± 0.02 μm vs 0.05 ± 0.02; n = 4; p<0.05). High magnification images from stressed rats showed an important loss of contact between cells, which was only maintained in the desmosome region (fig 7).
Figure 6 Effect of acute stress on the intercellular spaces of the oesophageal epithelium. Representative photomicrographs of the oesophageal epithelium in (A) control rat, (B) stressed rat, (C) control rat exposed to acid‐pepsin, and (D) stressed rat exposed to acid‐pepsin. An increase in the intercellular spaces was observed in stressed rats (B, D). Scale bars = 2.5 μm.
Figure 7 Transmission electron microscopy micrographs of rat oesophageal epithelium in (A) control rat and (B) stressed rat. An important loss of contact between cells only maintained in the desmosome region (arrows) was observed in stressed rats (B). Microvillous processes (mark) . Scale bars = 1 μm.
Discussion
Heartburn is thought to occur when refluxed gastric contents activate sensory nerve endings in the oesophageal wall. In patients with oesophagitis, acid and other components of gastric contents can reach sensory nerve endings through both the erosive and non‐erosive areas.2,28 In patients with non‐erosive GERD, DIS might facilitate acid to reach mucosal chemoreceptors.4 In both situations, the perception of symptoms can be modified by several factors, including the total surface and duration of mucosal exposure, the acidity of the refluxate, and extraoesophageal modulating factors such as duodenal contents or stress.29
Up to 60% of patients with GERD report an increase in symptoms related to stressful life events.9,10,30,31 Fass et al., using a dichotomous listening task, demonstrated that acute laboratory stress increased sensitivity to oesophageal acid exposure in patients with both erosive and non‐erosive reflux disease.32 The most accepted hypothesis to explain such stress‐increased heartburn is the occurrence of visceral hyperalgesia through central nervous system mechanisms of hypersensitivity.33,34
Because stress has been shown to increase mucosal permeability in other areas of the gastrointestinal tract13,14,18,19 and in the skin,20 we hypothesized that stress could also affect the oesophageal epithelium. Our experiments in rats showed that acute stress, by itself, increased oesophageal mucosa permeability and enlarged intercellular spaces. The combination of previous stress and mucosal exposure to acid‐pepsin induced the maximal increase in oesophageal mucosa permeability. These results suggest that there is a potential for stress to enhance the perception of heartburn (in humans) through a different peripheral mechanism—that is, increased exposure of oesophageal sensory nerve endings to gastric contents.
The experimental model of partial restraint used in the present study involves elements of physical stress in addition to psychological stress.24 This model has been previously used in studies on colonic visceral hypersensitivity.35 Partial restraint in rats is a mild and non‐ulcerogenic stressor22 that activates the HPA axis, resulting in increased plasma corticosterone levels.23
The functional integrity of the epithelial barrier can be assessed by measuring mucosal permeability in vitro to hydrophilic compounds of variable molecular weight and diameter, such as 51Cr‐EDTA36 and FITC‐labelled dextrans.3 It is generally accepted that trans‐epithelial movement of these molecules occurs as a result of passive diffusion through the paracellular (intercellular) pathway.37,38
Oesophageal epithelial resistance to luminal acid has been extensively studied by Orlando et al. in a rabbit oesophageal mucosa model.39 Prolonged contact with luminal acid and acid‐pepsin alters the properties of the intercellular junctions, which increases paracellular permeability to FITC‐dextran molecules,3 thereby enabling acid influx into the intercellular space and subsequent mucosal acidification.
In both animal models and humans, oesophageal acid exposure is associated with DIS.1,2,28,40 This feature has been observed by pathologists for many years using both light microscopy and electron microscopy; however, the subject only recently resurged and has been quantified because of its possible role in the pathophysiology of non‐erosive GERD.2,40,41 When considering the relationship between permeability and DIS, however, it should be noticed that increases of oesophageal mucosal permeability to molecules of a diameter of 2–8 nanometers38 may well occur before any observed DIS, which is defined as intercellular spaces larger than 1.5 μm.40
In contrast to previous findings in rabbit and humans,3,6 exposing mucosa from non‐stressed rats to identical concentrations of acid‐pepsin neither increased permeability to any molecule nor provoked DIS. These divergent results could be due to species differences. Rat oesophageal mucosa is covered by a thin keratin layer. However, the keratin layer observed in rats could not prevent the increased passage of protons through the oesophageal mucosa after sialonadectomy.42 Interestingly, if the rat oesophageal mucosa is more protected against acid injury than rabbit or humans, this effect is completely overcome by acute stress or stress followed by acid‐pepsin exposure.
In the stomach,13,14 small bowel18 and colon,19 stress increases mucosal permeability. In the skin, stress can alter cutaneous permeability by decreasing corneodesmosomes.20 The present study shows, for the first time, that stress, by itself, can also increase oesophageal mucosa permeability and can enlarge the intercellular spaces in the basal layers. Stress increased rat oesophageal mucosa permeability only to the smallest molecule 51Cr‐EDTA (400 Da); however, this change could theoretically be enough to allow mucosal permeation to acid.
Stress‐induced permeability changes in the oesophagus were substantially smaller and had a different kinetics than those observed in the intestine. This can be explained by differences in exposed mucosal area and type of epithelium (squamous stratified vs monolayer intestinal villi).
The mechanisms underlying stress‐induced changes in oesophageal mucosal permeability are unknown. It has been shown that stress induces changes in intestinal permeability involving mast‐cell degranulation.18 A similar mechanism has been found in stress experiments provoking DIS in the mucosa of rat urinary bladder.43,44 Mast cells are present in rat oesophageal mucosa,45 and immobilization stress induces mast‐cell degranulation in the rat skin (stratified epithelium).46 We found an increased number of submucosal mast cells in our stressed rats. We speculate that stress induced increased oesophageal mucosa permeability and enlargement of intercellular spaces might be due to the modification or redistribution of tight junctions and/or desmosomes, which may be related to mast‐cell degranulation. Further studies will be required to elucidate this mechanism.
The combination of previous stress and mucosal exposure to acid‐pepsin induced enlargement of intercellular spaces and maximal increases in oesophageal mucosa permeability to small and median molecules. This effect contrasted with the lack of changes observed after acid‐pepsin exposure alone. A potentiation between stress and acid‐pepsin is commonly described in the pathogenesis of gastro‐duodenal ulcers in which the primary event is disruption of mucosal integrity, that can be due to stress, followed by acid penetration in the epithelium and ulcer production.15 Based on our findings in rat, we could speculate with the following sequence: stress induces enlargement of intercellular spaces and increased oesophageal mucosa permeability that permits the passage of acid through the epithelium which, in turn, activates a mechanism (ie, mast‐cell degranulation47 and creates alterations in the location and/or expression of tight junction proteins48) that further increases permeability to larger molecules.
The findings of the present study may be relevant for our understanding of symptom generation in GERD patients. Several studies have indicated a relationship between stress and heartburn severity,9,30,31 although the mechanisms are still unclear. There is no clear relationship between objective measurements of increased luminal‐acid exposure and intensity of symptom perception. Acute stress does not increase oesophageal acid exposure.9 Moreover, more than 50% of patients with non‐erosive symptomatic GERD have normal oesophageal acid exposure.30
An alternative explanation could be the presence of visceral hyperalgesia. This mechanism implies enhanced perception of peripheral stimulation, which can result from sensitisation at the level of sensory neurons innervating the oesophagus, but also from abnormal processing or modulation of visceral sensory information at the level of the central nervous system (spinal cord or brain).49 Because of the frequent dissociation between luminal acid exposure and symptom perception, and the influence of stress factors on heartburn perception, the central hypersensitivity hypothesis is currently the most accepted one. Observations of increased perception of acid reflux in subjects with greater anxiety9 and of increased sensitivity to oesophageal acid exposure during acute laboratory stress in GERD patients,32 all seem supportive of mechanisms of central sensitisation.
The results of the present study, however, suggest that stress has the potential to enhance the perception of acid exposure by a peripheral mechanism of increased mucosal permeability. Stress‐induced mucosal permeability, which allows increased exposure of oesophageal sensory nerve endings to refluxed gastric contents, could contribute to the exacerbation of symptoms both in erosive and non‐erosive GERD patients with otherwise normal luminal oesophageal acid exposure.
In conclusion, the present study shows that acute stress can, by itself, enlarge oesophageal mucosa intercellular spaces and increase its permeability. Stress can potentiate the effect of acid‐pepsin on the oesophageal mucosa by further increasing the permeability. This mechanism might contribute to stress influences on heartburn symptoms in humans.
Acknowledgements
We would like to thank Jos Van Pelt for his technical assistance. This work was supported by a “Geconcerteerde Onderzoeksactie” Grant from the Catholic University of Leuven, Belgium.
Abbreviations
DIS - dilated intercellular spaces
GERD - gastroesophageal reflux disease
HPA - hypothalamic‐pituitary‐adrenal
KHBB - Krebs–Henseleit bicarbonate buffer
PRS - Partial Restraint Stress
TEM - transmission electron microscopy
Footnotes
Statement of competing interests: None to declare.
References
- 1.Caviglia R, Ribolsi M, Maggiano N.et al Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure. Am J Gastroenterol 2005100543–548. [DOI] [PubMed] [Google Scholar]
- 2.Hopwood D, Milne G, Logan K R. Electron microscopic changes in human oesophageal epithelium in oesophagitis. J Pathol 1979129161–167. [DOI] [PubMed] [Google Scholar]
- 3.Tobey N A, Hosseini S S, Argote C M.et al Dilated intercellular spaces and shunt permeability in nonerosive acid‐damaged esophageal epithelium. Am J Gastroenterol 20049913–22. [DOI] [PubMed] [Google Scholar]
- 4.Barlow W J, Orlando R C. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology 2005128771–778. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo J, Hernandez C J, Vidal M A.et al Vegetative innervation of the esophagus. III. Intraepithelial endings. Acta Anat 197592242–258. [DOI] [PubMed] [Google Scholar]
- 6.Bateson M C, Hopwood D, Milne G.et al Oesophageal epithelial ultrastructure after incubation with gastrointestinal fluids and their components. J Pathol 198113333–51. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese C, Bortolotti M, Fabbri A.et al Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol 2005100537–542. [DOI] [PubMed] [Google Scholar]
- 8.Fass R. Epidemiology and pathophysiology of symptomatic gastroesophageal reflux disease. Am J Gastroenterol 200398S2–S7. [DOI] [PubMed] [Google Scholar]
- 9.Bradley L A, Richter J E, Pulliam T J.et al The relationship between stress and symptoms of gastroesophageal reflux: the influence of psychological factors. Am J Gastroenterol 19938811–19. [PubMed] [Google Scholar]
- 10.Naliboff B D, Mayer M, Fass R.et al The effect of life stress on symptoms of heartburn. Psychosom Med 200466426–434. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Aziz Q, Woolf C J.et al Contribution of central sensitisation to the development of non‐cardiac chest pain. Lancet 20003561154–1159. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar S, Hobson A R, Furlong P L.et al Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2001281G1196–G1202. [DOI] [PubMed] [Google Scholar]
- 13.Coskun T, Yegen B C, Alican I.et al Cold restraint stress‐induced gastric mucosal dysfunction. Role of nitric oxide. Dig Dis Sci 199641956–963. [DOI] [PubMed] [Google Scholar]
- 14.Meddings J B, Swain M G. Environmental stress‐induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 20001191019–1028. [DOI] [PubMed] [Google Scholar]
- 15.Peterson W L. The role of acid in upper gastrointestinal haemorrhage due to ulcer and stress‐related mucosal damage. Aliment Pharmacol Ther 19959(Suppl 1)43–46. [DOI] [PubMed] [Google Scholar]
- 16.Werther J L, Horowitz I. The effect of stress on the gastric mucosal barrier in rats. Proc Soc Exp Biol Med 1977154415–417. [DOI] [PubMed] [Google Scholar]
- 17.Mazzon E, Sturniolo G C, Puzzolo D.et al Effect of stress on the paracellular barrier in the rat ileum. Gut 200251507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos J, Benjamin M, Yang P C.et al Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol 2000278G847–G854. [DOI] [PubMed] [Google Scholar]
- 19.Saunders P R, Santos J, Hanssen N P.et al Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci 200247208–215. [DOI] [PubMed] [Google Scholar]
- 20.Choi E H, Brown B E, Crumrine D.et al Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol 2005124587–595. [DOI] [PubMed] [Google Scholar]
- 21.Gue M, Del Rio‐Lacheze C, Eutamene H.et al Stress‐induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 19979271–279. [DOI] [PubMed] [Google Scholar]
- 22.Williams C L, Villar R G, Peterson J M.et al Stress‐induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology 198894611–621. [DOI] [PubMed] [Google Scholar]
- 23.Strausbaugh H J, Dallman M F, Levine J D. Repeated, but not acute, stress suppresses inflammatory plasma extravasation. Proc Natl Acad Sci USA 19999614629–14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soderholm J D, Perdue M H. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 2001280G7–13. [DOI] [PubMed] [Google Scholar]
- 25.Barone F C, Deegan J F, Price W J.et al Cold‐restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol 1990258G329–G337. [DOI] [PubMed] [Google Scholar]
- 26.Marquez C, Belda X, Armario A. Post‐stress recovery of pituitary‐adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Res 2002926181–185. [DOI] [PubMed] [Google Scholar]
- 27.Shahana S, Jaunmuktane Z, Stenkvist A M.et al Ultrastructural investigation of epithelial damage in asthmatic and non‐asthmatic nasal polyps. Respir Med 20061002018–2028. [DOI] [PubMed] [Google Scholar]
- 28.Bove M, Vieth M, Dombrowski F.et al Acid challenge to the human esophageal mucosa: effects on epithelial architecture in health and disease. Dig Dis Sci 2005501488–1496. [DOI] [PubMed] [Google Scholar]
- 29.Fass R, Tougas G. Functional heartburn: the stimulus, the pain, and the brain. Gut 200251885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fass R, Fennerty M B, Vakil N. Nonerosive reflux disease‐current concepts and dilemmas. Am J Gastroenterol 200196303–314. [DOI] [PubMed] [Google Scholar]
- 31.Gallup A Gallup Organization National Survery: Heartburn across America. NJ: Princeton, 1988
- 32.Fass R, Malagon I, Naliboff B D.et al Effect of psychologically induced stress on symptom perception and autonomic nervous system response of patients with erosive esophagitis (EE) and non‐erosive reflux disease (NERD). Gastroenterology 2000118A637 [Google Scholar]
- 33.Fass R, Dickman R. Non‐cardiac chest pain: an update. Neurogastroenterol Motil 200618408–417. [DOI] [PubMed] [Google Scholar]
- 34.Mayer E A, Gebhart G F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994107271–293. [DOI] [PubMed] [Google Scholar]
- 35.Mayer E A, Collins S M. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology 20021222032–2048. [DOI] [PubMed] [Google Scholar]
- 36.Benjamin M A, McKay D M, Yang P C.et al Glucagon‐like peptide‐2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 200047112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjarnason I, Macpherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 19951081566–1581. [DOI] [PubMed] [Google Scholar]
- 38.Hoogstraate A J, Cullander C, Nagelkerke J F.et al Diffusion rates and transport pathways of fluorescein isothiocyanate (FITC)‐labeled model compounds through buccal epithelium. Pharm Res 19941183–89. [DOI] [PubMed] [Google Scholar]
- 39.Orlando R C, Lacy E R, Tobey N A.et al Barriers to paracellular permeability in rabbit esophageal epithelium. Gastroenterology 1992102910–923. [DOI] [PubMed] [Google Scholar]
- 40.Tobey N A, Carson J L, Alkiek R A.et al Dilated intercellular spaces: a morphological feature of acid reflux‐damaged human esophageal epithelium. Gastroenterology 19961111200–1205. [DOI] [PubMed] [Google Scholar]
- 41.Villanacci V, Grigolato P G, Cestari R.et al Dilated intercellular spaces as markers of reflux disease: histology, semiquantitative score and morphometry upon light microscopy. Digestion 2001641–8. [DOI] [PubMed] [Google Scholar]
- 42.Sarosiek J, Feng T, McCallum R W. The interrelationship between salivary epidermal growth factor and the functional integrity of the esophageal mucosal barrier in the rat. Am J Med Sci 1991302359–363. [DOI] [PubMed] [Google Scholar]
- 43.Ercan F, San T, Cavdar S. The effects of cold‐restraint stress on urinary bladder wall compared with interstitial cystitis morphology. Urol Res 199927454–461. [DOI] [PubMed] [Google Scholar]
- 44.Ercan F, Akici A, Ersoy Y.et al Inhibition of substance P activity prevents stress‐induced bladder damage. Regul Pept 200613382–89. [DOI] [PubMed] [Google Scholar]
- 45.Majeed S K. Mast cell distribution in rats. Arzneimittelforschung 199444370–374. [PubMed] [Google Scholar]
- 46.Singh L K, Pang X, Alexacos N.et al Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav Immun 199913225–239. [DOI] [PubMed] [Google Scholar]
- 47.Paterson W G. Role of mast cell‐derived mediators in acid‐induced shortening of the esophagus. Am J Physiol 1998274G385–G388. [DOI] [PubMed] [Google Scholar]
- 48.Asaoka D, Miwa H, Hirai S.et al Altered localization and expression of tight‐junction proteins in a rat model with chronic acid reflux esophagitis. J Gastroenterol 200540781–790. [DOI] [PubMed] [Google Scholar]
- 49.Jones M P, Dilley J B, Drossman D.et al Brain‐gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil 20061891–103. [DOI] [PubMed] [Google Scholar]