The occurrence of glucocorticoid dependent or refractory disease courses is a major clinical problem not only in inflammatory bowel disease (IBD) but also in other chronic inflammatory conditions such as asthma and rheumatoid arthritis.1 It would be of great value if the responsiveness of patients to glucocorticoids could be evaluated before their administration to avoid ineffective treatment.
The response to steroids is mediated by the glucocorticoid receptor (hGR) of which two isoforms exist, hGRα and hGRβ. Both are products of alternative splicing of the primary transcript of GR messenger RNA (mRNA).2 The hGR isoforms differ in their carboxyl termini: whereas hGRα is ligand activated, modulating the expression of glucocorticoid responsive genes by binding to specific glucocorticoid response elements (GREs), hGRβ does not bind glucocorticoids and is transcriptionally inactive. Therefore it has been suggested that hGRβ might be an endogenous inhibitor of glucocorticoid action and a negative regulator determining glucocorticoid sensitivity in the target tissues.3
In 2000 Honda and coworkers published a study indicating that hGRβ mRNA was detectable in peripheral blood mononuclear cells (PBMC) in 9.1% of cases of glucocorticoid sensitive ulcerative colitis, 83.3% of cases of glucocorticoid resistant ulcerative colitis, and 10% of healthy volunteers.4 The authors concluded that the expression of hGRβ mRNA in PBMC could serve as a novel predictor of glucocorticoid response in patients with ulcerative colitis or IBD in general.
As the prediction of steroid response is an important issue in the clinical management of IBD, we prospectively and retrospectively tested this hypothesis in a cohort of patients by quantitative polymerase chain reaction (PCR). Blood samples were collected from 21 healthy controls, 16 newly diagnosed steroid naive IBD patients, 35 IBD patients in remission treated with or without glucocorticoids, and 35 patients with active disease. RNA was isolated using the RNeasy® Mini Kit (Qiagen, Hilden, Germany). mRNA was reverse transcribed (Promega, Madison, USA) in a 15 minute reaction at 42°C. hGRα/GAPDH and hGRβ/GAPDH mRNA ratio levels were determined from isolated PBMC using quantification of gene expression by Taqman PCR (hGRα probe: 5′‐TTG GAT AAG ACC ATG AGT ATT GAA TTC C‐3′, hGRα forward: 5′‐TCT CCT TAA CTA TTG CTT CCA AAC ATT‐3′, hGRα reverse 5′‐TGG TGA TGA TTT CAG CTA ACA TCT C‐3′, hGRβ probe: 5′‐TGG CGC TCA AAA AAT AGA ACT CAA TGA GAA AA‐3′, hGRβ forward: 5′‐TTA ATC TGA TTT TCA TCC CAA CAA TC‐3′, hGRβ reverse: 5′‐TTG ACA ACG AAG TGC ACA TAA TCT T‐3′; human GAPDH: No 4310884E, Applied Biosystems, Warrington, UK). Clinical response (disease course: remission, active disease; side effects) to steroid treatment was determined by medical record review and was correlated with hGRα and hGRβ levels.
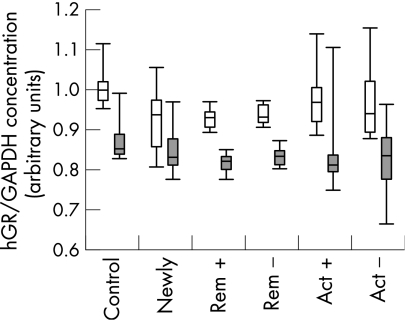
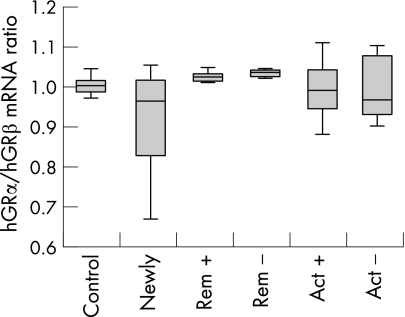
In the setting described, hGRβ was detected in all samples investigated at similar levels, indicating that an expression or significant upregulation only in glucocorticoid refractory patients is unlikely (fig 1). The ratio between the systemic (PBMC) hGRα and hGRβ levels of steroid treated IBD patients, IBD patients not receiving steroid treatment, and the control subjects did not differ significantly (fig 2). Steroid treatment was not inversely correlated with hGR mRNA expression, as it was supposed to be from earlier data on reduced glucocorticoid binding in treated patients.5 Obviously, glucocorticoid binding capacity cannot simply be estimated from mRNA expression of hGR. Ten of the patients who presented with active IBD could not be treated sufficiently with steroids and had to be switched to other immunosuppressive agents, indicating steroid refractory or steroid dependent disease. There was no significant difference in the other patient groups with respect to their ratio of hGRα to hGRβ mRNA levels. Unfortunately, among the 12 patients evaluated prospectively, only one turned out to be steroid dependent and one steroid resistant in a two year follow up, allowing no statistical analysis. However, these two prospectively evaluated patients with impaired steroid response did not differ in their hGRβ mRNA expression from the steroid responders.
Figure 1 hGRα (white bars) and hGRβ (grey bars) mRNA expression in peripheral blood mononuclear cells from healthy staff members (control, n = 21), newly diagnosed steroid naive IBD patients (n = 16), IBD patients in remission treated with (rem +, n = 15) or without glucocorticoids (rem −, n = 20) and patients with active disease with (act +, n = 16) or without glucocorticoids (act −, n = 19). IBD, inflammatory bowel disease.
Figure 2 Ratio of hGRα to hGRβ mRNA expression in peripheral blood mononuclear cells from healthy staff members (control, n = 21), newly diagnosed steroid naive IBD patients (n = 16), IBD patients in remission treated with (rem +, n = 15) or without glucocorticoids (rem −, n = 20) and patients with active disease with (act +, n = 16) or without glucocorticoids (act −, n = 19). IBD, inflammatory bowel disease.
Our findings do not exclude the possibility that hGR protein levels may differ in the respective groups as a result of post‐transcriptional regulation. However, quantification of hGR protein levels is difficult and does not provide evidence for the amount of “free” receptor with the ability to bind steroids. Nevertheless, our data indicate that the ratio of PBMC hGRα to hGRβ mRNA expression is not correlated with effective glucocorticoid treatment in IBD. Therefore, we conclude—in contrast to Honda and coworkers—that hGRβ expression has no predictive value for the efficacy of steroid treatment.
Acknowledgements
We thank Sabine Fink for technical assistance.
Footnotes
Conflict of interest: None declared.
References
- 1.Sandborn W J. Steroid‐dependent Crohn's disease. Can J Gastroenterol 200014(suppl C)17–22C. [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg S M, Weinberger C, Ong E S.et al Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985318635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamberger C M, Bamberger A M, de Castro M.et al Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest 1995952435–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda M, Orii F, Ayabe T.et al Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid‐resistant ulcerative colitis. Gastroenterology 2000118859–866. [DOI] [PubMed] [Google Scholar]
- 5.Rogler G, Meinel A, Lingauer A.et al Glucocorticoid receptors are down‐regulated in inflamed colonic mucosa but not in peripheral blood mononuclear cells from patients with inflammatory bowel disease. Eur J Clin Invest 199929330–336. [DOI] [PubMed] [Google Scholar]