Abstract
Background/Aims
Urocortin II (UcnII) is a neuropeptide that binds with high affinity to the corticotropin‐releasing hormone receptor 2 (CRHR2) in peripheral tissues. UcnII is synthesised in the intestine, but its role in human intestinal inflammation is largely unknown.
Methods
Responses of human colonic epithelial cells expressing CRHR2 to stimulation by UcnII were measured using ELISA, western blot analysis, real‐time reverse transcription‐PCR (RT‐PCR) and interleukin (IL)8 promoter activity. Expression levels of CRHR2 and UcnII in human colitis were determined by immunofluorescence and real‐time RT‐PCR in mucosal biopsies from patients with Crohn's and ulcerative colitis, and in human intestinal xenografts after exposure to Clostridium difficile toxin A.
Results
It is reported here that expression of CRHR2 mRNA and protein in human colonic epithelial cells (HT‐29) are increased by exposure to C difficile toxin A or tumour necrosis factor (TNF)α. Stimulation of non‐transformed NCM460 colonocytes overexpressing CRHR2α receptor with UcnII resulted in a time‐ and concentration‐dependent increase in IL8 production. UcnII stimulation also led to activation of nuclear factor‐κB (NF‐κB) and mitogen‐acivated protein (MAP) kinase in these cells, as evidenced by degradation of IκBα and phosphorylation of the p65 subunit of NF‐κB and extracellularly regulated kinase (ERK) 1/2. Furthermore, expression of UcnII and CRHR2 mRNA was increased in mucosal samples of patients with inflammatory bowel disease, and after exposure of human intestinal xenografts to C difficile toxin A.
Conclusions
These results suggest that UcnII has pro‐inflammatory effects in human intestinal cells via the CRHR2α receptor and may play an important role in the pathophysiology of colitis in humans.
Corticotropin‐releasing hormone (CRH), and urocortins (Ucns) I, II and III, are structurally related neuropeptides distributed throughout the body that participate in stress and immune responses.1 All members of this family bind to one or both of two G‐coupled transmembrane receptors, CRHR1 and CRHR2.2 UcnII is a 38 amino acid peptide expressed in the stomach, small bowel and colon3,4,5 that binds CRHR2 specifically.4 Although the regulation of UcnII expression in the intestinal tract is unknown, UcnII mRNA levels were found to be decreased in other epithelial tissues after administration of dexamethasone.6 The peripheral effects of UcnII are diverse, including inhibition of gastric emptying, impairment of host resistance to bacterial infection and induction of macrophage apoptosis.7,8,9 Little is known about the effects of UcnII on gut inflammation per se, apart from a study by La Fleur et al, who showed that inhibition of UcnII expression by double‐stranded RNA in the rat ileum did not alter the inflammatory responses to Clostridium difficile toxin A.5
Three isoforms of CRHR2 have been described, α, β and γ.10 The α isoform is expressed throughout the brain and peripheral tissues, whilst the β and γ isoforms are only expressed in specific regions of the brain in humans.11,12 Human colonic epithelial HT‐29 cells express CRHR2α mRNA, while expression of the β and γ splice variants is minimal.13 The signalling pathways linked to CRHR2 in respect to inflammation have not been well studied. CRHR2 has been shown to be linked to interleukin (IL)6 production when stimulated by UcnII in aortic smooth muscle cells.14
We have previously shown that ileal administration of C difficile toxin A to mice led to increased CRHR2 expression15 and that a specific CRHR2 antagonist reduces the secretory and inflammatory responses to this toxin.13 We also showed that only UcnII, but not other Ucns, was significantly upregulated in the mouse ileum following toxin A exposure.13 Moreover, treatment of HT‐29 colonic adenocarcinoma epithelial cells with UcnII stimulated expression of the potent chemoattractants IL8 and monocyte chemoattractant protein‐1 (MCP‐1).13 However, the pro‐inflammatory signalling pathways activated in UcnII‐exposed colonocytes are not known, and whether CRHR2 and UcnII expression is altered in human colitis has not been examined. The purpose of this study was to elucidate the pro‐inflammatory mechanisms of UcnII in human colonocytes in vitro and to study expression of both UcnII and CRHR2 in human colitis of different aetiologies.
Materials and methods
Reagents
Recombinant human tumour necrosis factor (TNF)α was purchased from R&D Systems (Minneapolis, MN, USA). Toxin A was purified to homogeneity from C difficile strain 10465 cultures and tested for cytotoxicity and enterotoxicity as previously described.16 Astressin 2B was kindly provided by Neurocrine Biosciences (San Diego, CA, USA). Previous studies indicated the specificity of this peptide antagonist for CRHR2 and its ability to alter CRH‐ or Ucn‐associated responses in vivo.17,18 Antibodies against phospho p65, phospho extracellularly regulated kinase (ERK) 1/2, phospho p38, phospho JNK and the MEK 1/2‐specific inhibitor PD98059 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against IκBα and glyceraldehyde phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell lines
The human colonic epithelial cell line HT‐29 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in McCoy's 5A medium supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic (Invitrogen, Carlsbad, CA, USA) at 37°C in a 95% humid atmosphere, with 5% CO2. The non‐transformed colonic epithelial NCM460 cells were purchased from INCELL Corporation (San Antonio, TX, USA) and cultured in M3D media supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic, as previously described.19
NCM460 cells were stably transfected using lipofectamine with a plasmid containing the human CRHR2α cDNA cloned in pDNA3 (UMR cDNA Resource Center, University of Missouri‐Rolla), and cells carrying the plasmid were selected by treatment with geneticin (0.5%) (Invitrogen Corporation, Carlsbad, CA, USA). Individual clones were picked up from foci and continue to grown in the presence of geneticin. CRHR2 overexpression was verified by real‐time reverse transcription‐PCR (RT‐PCR) and western blot analyses as described below.
Cell stimulation
NCM460 cells overexpressing CRHR2α at 90% confluence were serum starved for 24 h before stimulation with 1× phosphate‐buffered saline (PBS; negative control), UcnII (107 M) (Bachem Bioscience, King of Prussia, PA, USA) or TNFα (10 ng/ml) as positive control. For inhibition experiments, astressin 2B (1 μM), PD98059 (25 μM) (Cell Signaling Technology, Beverly, MA, USA), MG 132 (25 μM) or SB203580 (20 μM) (Calbiochem, CA, USA) were used at concentrations previously reported to produce complete inhibition in epithelial cells.20,21,22,23,24,25 To determine the concentration response to UcnII, cells were stimulated as above with 10−6–10−10 M UcnII for 8 h. In separate experiments, HT‐29 cells were stimulated with toxin A (1 μg/ml), TNFα (10 ng/ml), UcnII (10−7 M) or buffer at 37°C. At specific time points after stimulation, supernatants were aspirated from each well and snap‐frozen at –80°C for ELISA, while the cells were lysed with either 100 μl of 3× SDS sample buffer (Cell Signalling Technology, Danvers, MA, USA) for protein extracts, or 500 μl of Trizol (Invitrogen) for RNA extraction before being frozen at –80°C.
Human tissues
Patients with inflammatory bowel disease (IBD) (n = 8) undergoing colonoscopy had biopsies taken from areas of normal mucosa and colitis that were snap‐frozen at –80°C. There were three patients with Crohn's colitis and five patients with ulcerative colitis in this cohort. For immunofluorescence, samples were frozen in OCT (Sakura Finetek, Torrance, CA, USA). All subjects gave informed consent, and the Beth Israel Deaconess Medical Center institutional review board approved the protocol. Each set of samples was categorised based on the patient's disease activity at the time of acquisition, and all samples used were obtained from patients with at least moderately active disease based on these indices.26,27 Each histologically involved and uninvolved region was confirmed as such by a pathologist. Tissue samples were disrupted and homogenised in Trizol using a rotor‐starter homogeniser (TissueMiser, Fisher Scientific, Pittsburgh, PA, USA) for RNA extraction.
Clostridium difficile toxin A treatment of human intestinal xenografts
Human fetal intestine was obtained from Brigham and Women's Hospital (Boston, MA, USA) (mean age (SD): 14.2 (1.6) weeks) after therapeutic abortions. Procurement and procedures involving xenografting were realised after full approval from the local ethics committee. Fetal small intestinal and colonic tissues were washed in Dulbecco's modified Eagle's medium (DMEM) and xenotransplanted subcutaneously into SCID mice as previously described.16 Twelve weeks after xenografting, the graft lumen was injected with 400 μl of PBS containing 10 μg of purified toxin A or medium control (n = 4 per group). Animals were killed after 6 h and the grafted tissues were removed for preparation of RNA and frozen sections.
Laser capture microdissection (LCM)
Frozen colonic xenograft sections (8 μm) were fixed in 70% ethanol, 95% ethanol, 100% ethanol and xylene with a final incubation in clean xylene for 10–20 min. Each section was then air dried and used immediately for LCM. Individual epithelial cells were removed from histological sections using a PixCell II LCM system (Arcturus, Mountain View, CA, USA). An estimated 600–1000 cells per tissue sample were captured onto CapSure IS ICM caps and RNA was prepared using a Picopore RNA isolation kit (Arcturus). RNA was treated with DNase I (Qiagen Inc., Valencia, CA, USA) and used to generate complementary DNA using a GeneAmp kit (Applied Biosystems, Foster City, CA, USA).
Quantification of gene expression
Total RNA was extracted from frozen tissues using the RNeasy kit supplemented with DNase treatment (Qiagen Inc.).
Competitive PCR for human CRHR2 in HT29 cells
A 2 μg aliquot of RNA was added to 1 μl of random hexamers (0.125 μg/μl) and RNase‐free water to a final volume of 10 μl in RNase‐free tubes. Tubes were then heated for 2 min at 70°C before adding 4 μl of RNase‐free water, 5 μl of 5× reverse transcription buffer, 2 μl of 10 mM dNTPs, 2 μl of dithiothreitol (DTT; 0.1 M) and 1 μl of Moloney murine leukaemia virus reverse transcriptase (200–400 U/μl). The reverse transcriptase reaction was incubated for 1 h at 37°C followed by 15 min at 70°C. The following primers were used to perform the PCR for human CRHR2 (all isoforms): forward, 5′‐GTC CTC CTC CAC CTT CTT‐3′; reverse, 5′‐ GGG TCC TGT GAA TTC CCT CT‐3′. The CRF2α, CRF2ß and CRF2γ exon‐specific primers were those published by Kostich et al11 The PCR was set up as competitive RT‐PCR using the 18S ribosomal subunit as an internal control (Quantum RNA 18S internal standards, Ambion Inc., Austin, TX, USA). The PCR conditions were: 5 min at 94°C followed by 33 cycles of 30 s at 94°C, 30 s at 58°C and 30 s at 72°C, and a final extention stage at 72°C for 7 min. Quantification of the PCR bands was performed by densitometry using Scion image analysis software (Scion Corporation, Frederick, MD, USA). This approach was used for the measurement of CRH2 expression in HT‐29 cells, and CRHR2 isoform expression in NCM460 cells.
Real‐time RT‐PCR for CRHR2, UncII and IL8
A 50 ng aliquot of RNA was used in a TaqMan One‐Step RT‐PCR reagent mixture including gene‐specific primers and a FAM‐labelled probe (Assays on Demand, Applied Biosystems). Reactions were run in duplicate in a 5700 Sequence Detection System (Applied Biosystems) and the results were expressed as arbitrary mRNA units normalised by TATA‐binding protein (TBP) expression. This approach was used for the IL8 and human and mouse CRHR2 and UcnII measurements, with the exception of experiments involving the HT‐29 cells.
Western blotting
Protein concentration was measured using the DC protein assay (Biorad, Hercules, CA, USA). For immunoblots, proteins (15–30 μg) were separated by electrophoresis in a 10% polyacrylamide gel. Protein samples were mixed with sample buffer (3×; Cell Signaling Technology) and denatured by boiling. Samples were electrophoresed at 100–150 V for 1.5 h or longer until the dye migrated to the bottom of the gel. The separating gel was equilibrated in transfer buffer (20 mM Tris–HCl/150 mM glycine/20% methanol/0.1% SDS) for 10 min. The proteins were then transferred to poly(vinylidene difluoride) membranes (PVDF, Millipore; Billerica, Massachusetts, USA) at 4°C. The membranes were blocked for 1 h at room temperature in blocking buffer (Tris‐buffered saline (TBS)/5% non‐fat dry milk (Bio‐Rad, Hercules, California, USA)/0.1% Tween‐20) and then incubated with primary antibodies overnight at 4°C. Horseradish peroxidase‐conjugated secondary antibodies in blocking buffer were used for 1 h at room temperature (Santa Cruz Biotechnology, Santa Cruz, California, USA). The proteins were visualised by using SuperSignal West Pico chemiluminescent substrate (Pierce). The membranes were exposed to x‐ray films from 10 s to 5 min.
Luciferase assay
The role of transcriptional regulation of IL8 by UcnII was examined using transient transfection with an IL8 promoter luciferase–reporter construct as previously described by us.19 NCM460 cells overexpressing CRHR2α were transfected with IL8 promoter–reporter vector, either intact or mutated at the nerve factor‐κB (NF‐κB)‐binding site19 using the Effectene transfection reagent method (Qiagen).19 Briefly, 2×10–5 cells per well in 6‐well plates were washed with 1× PBS. Each vector (0.5 μg), along with the pRL‐TK vector, was mixed with buffer EC, enhancer, Effectene reagent and medium (serum‐free), incubated at room temperature for 20 min, and added to the cells. The cells were washed again 16 h later. Fresh medium along with 10–7 M UcnII was added for 6 h, with or without astressin 2B (1 μM). Luciferase activity was determined using the dual‐luciferase reporter assay system (Promega, Madison, Wisconsin, USA). Firefly luciferase activity was expressed relative to Renilla luciferase activity. Each experimental condition was run in triplicate.
Immunoflouresence
Slides were thawed at room temperature for 60 min and then incubated in 80% acetone for 2 min at 4°C. Slides were washed three times in double‐distilled H2O. To block endogenous peroxidase activity, the slides were incubated in 3% hydrogen peroxide for 10 min. Slides were then washed in water twice and rinsed in TBS. Sections were incubated for 60 min at room temperature in a blocking solution consisting of 5% normal donkey serum in TBS. The primary antibody (goat anti‐CRHR2) was diluted in the blocking solution at a concentration of 1:50 and applied to the slides. For a negative control, the right half of each slide was incubated in only the blocking solution. Slides were incubated overnight at 4°C. Primary antibody was removed and the sections were washed three times in TBS. Fluorescein isothiocyanate (FITC)‐conjugated donkey anti‐goat secondary antibody was diluted at a concentration of 1:200 in TBS, applied to the slides and incubated for 60 min at room temperature. Slides were then washed three times in TBS. Slides were coverslipped with Vectashield mounting medium for fluorescence and stored at –20°C.
ELISA
IL‐8 was measured in cell supernatants by ELISA (DuoSet, R&D Systems, Minneapolis, MN, USA). Briefly, 50 μl of undiluted supernatant from treated cells was added to a 96‐well plate prepared with capture antibody as per the manufacturer's directions. IL8 concentrations were calculated based on optical density at 480 nm in a microplate reader.
Statistical analysis
Results were expressed as mean (SEM) and the data analysed using the StatView statistical software program (SAS, Cary, NC, USA). An analysis of variance (ANOVA) was performed with the appropriate corrections for multiple comparisons. A p value of <0.05 was considered statistically significant for multiple comparisons.
Results
Clostridium difficile toxin A and TNFα stimulate CRHR2 expression in HT‐29 colonocytes
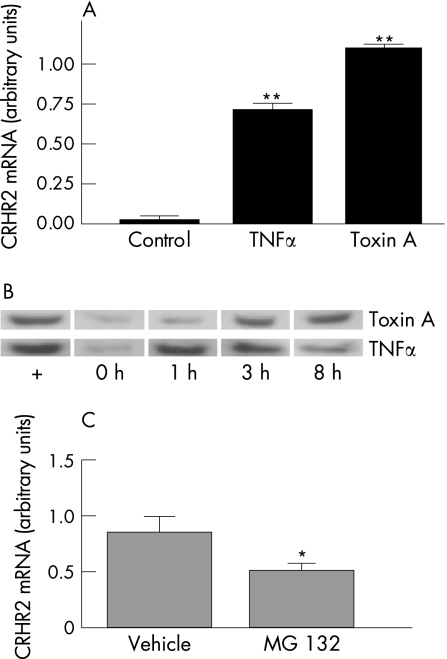
Previous studies showed increased CRHR2 mRNA expression in the intestinal mucosa in response to toxin A administration in vivo.15 We have also reported the presence of CRHR2α in colonic adenocarcinoma HT‐29 cells.13 However, whether toxin A or the pro‐inflammatory cytokine TNFα, which is associated with toxin A enteritis,28 can directly stimulate expression of CRHR2 in colonocytes has not been examined. To address this, we exposed HT‐29 cells to purified toxin A and TNFα, and determined expression of CRHR2 mRNA and protein in these cells. We found that CRHR2 mRNA expression was significantly increased by both toxin A and TNFα at 3 h (p<0.001, fig 1A). We subsequently prepared cytosolic extracts from toxin A‐ or TNFα‐exposed HT‐29 cells and examined CRHR2 protein expression in equal amounts of total protein by western blot analysis using an antibody specific for human CRHR2. Unstimulated human umbilical vein endothelial cells (HUVECs), known to express CRHR2,29 were used as positive control. Consistent with our previous results,13 we found that HT‐29 cells express CRHR2 protein (fig 1B). However, exposure to toxin A resulted in increased CRHR2 protein levels at 3 h, and a similar increase after 1 h of TNFα exposure (fig 1B).
Figure 1 Clostridium difficile toxin A and tumour necrosis factor (TNF)α increase corticotropin‐releasing hormone receptor 2 (CRHR2) mRNA and protein expression in HT‐29 colonic epithelial cells. (A) CRHR2 mRNA expression as measured by competitive PCR in HT‐29 cells incubated with toxin A (1 μg/ml) or TNFα (10 ng/ml) for 3 h (**p<0.001). (B) Western blot analysis for CRHR2 protein in HT‐29 cells incubated with toxin A (0.3 μg/ml) or TNFα (10 ng/ml) for 1, 3 and 8 h. (C) CRHR2 mRNA expression as measured by real‐time reverse transcription‐PCR (RT‐PCR) in HT‐29 cells incubated with toxin A (0.3 μg/ml) for 3 h following 1 h pre‐incubation with vehicle or the proteasome inhibitor MG 132 (CRHR2 mRNA expression normalised to glyceraldehyde phosphate dehydrogenase, *p<0.05).
Since toxin A is a potent activator of the NF‐κB pathway in HT‐2930 and other cells,31,32 we also examined whether this transcription factor is involved in toxin A‐induced upregulation of CRHR2. HT‐29 cells were incubated with the specific proteasome inhibitor, MG 132, that blocks NF‐κB activation, or its vehicle, dimethylsulphoxide (DMSO), and then stimulated with toxin A. One‐hour pre‐incubation of HT29 cells with 25 μM MG 132 significantly reduced CRHR2 mRNA expression after 3 h of toxin A exposure (p<0.01, fig 1C).
UcnII stimulates chemokine expression in human colonic epithelial cells via CRHR2α
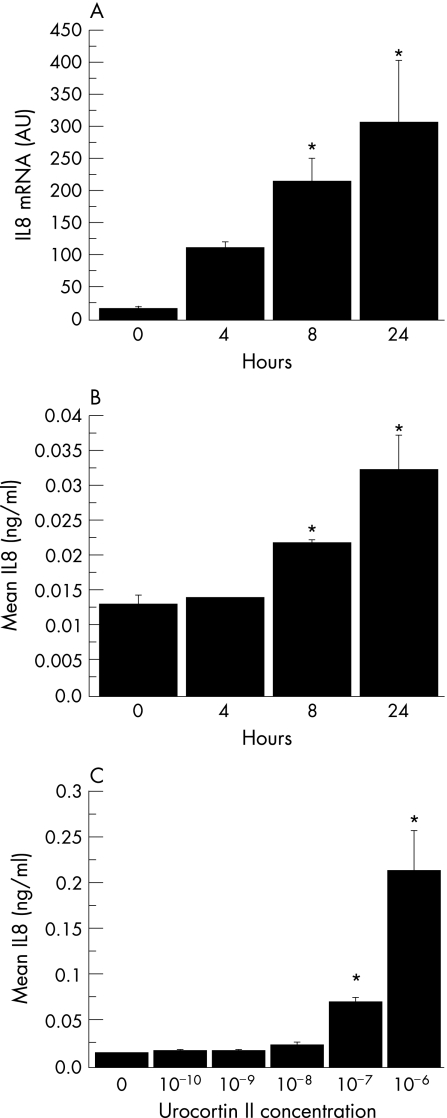
Since UcnII is a specific ligand for CRHR2, we examined its effect on CRHR2 in human colonocytes. To use a more “physiological” colonic epithelial cell line, we performed all remaining experiments in NCM460 colonocytes, previously shown to have a non‐transformed phenotype.33 In preliminary real‐time RT‐PCR experiments, we determined that NCM460 cell express CRHR2α mRNA, albeit at low levels (data not shown). Next, we generated NCM460 cells stably transfected with CRHR2α and confirmed increased expression of CRHR2α mRNA and protein by real‐time RT‐PCR and western blot analysis, respectively (data not shown). Stimulation of these cells with UcnII (10−7 M) led to a time‐dependent increase in IL8 mRNA and protein expression, as measured by both RT‐PCR and ELISA, which was statistically significant 8 h after stimulation (p<0.05, fig 2A and B). This response did not occur in non‐transfected cells (data not shown). Increased IL8 production was evident at UcnII concentrations higher than 10−8 M (p<0.05, fig 2C).
Figure 2 Urocortin II (UcnII) stimulates chemokine expression in non‐transformed human colonic epithelial NCM460 cells via corticotropin‐releasing hormone receptor 2α (CRHR2α). (A) Interleukin (IL)8 mRNA expression as measured by real‐time PCR in NCM460 cells. NCM460 cells stably transfected with CRHR2α, after stimulation with UcnII (10−7 M). NCM460 cells stably transfected with CRHR2α were serum starved for 24 h and then stimulated with UcnII (10−7 M) for the indicated times. Results have been normalised to TATA‐binding protein (*p<0.05). (B) IL8 concentration (ng/ml) was determined using a human IL8 ELISA assay in NCM460/CRHR2α cells stimulated with UcnII (10−7 M). NCM460 cells stably transfected with CRHR2α for the indicated times (*p<0.05). (C) IL8 concentration (ng/ml) in cell culture supernatants was determined using a human IL8 ELISA assay in NCM460/CRHR2α cells stimulated with UcnII at various concentrations for 24 h (* p<0.05).
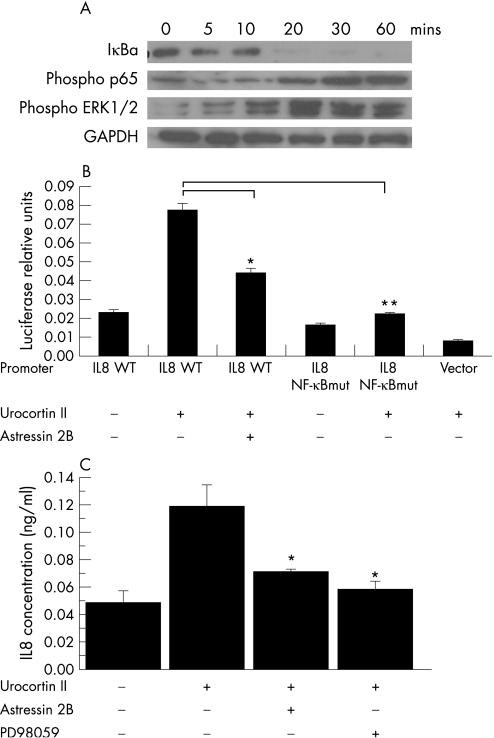
To determine the signalling pathways involved in UcnII‐induced increased IL8 expression, we examined the effect of UcnII on NF‐κB and mitogen‐activated protein (MAP) kinase pathways in CRHR2α‐overexpressing NCM460 cells. Stimulation with UcnII (10−7 M) led to early (20 min) degradation of IκBα and phosphorylation of the NF‐κB p65 subunit, suggesting activation of the NF‐κB pathway (fig 3A). In addition, UcnII led to phosphorylation of the ERK 1/2 MAP kinase at 10 min after stimulation (fig 3A). In contrast, UcnII did not cause phosphorylation of p38 or JNK in these cells. Moreover, pharmacological inhibition of p38 by SB203580 did not reduce UcnII‐induced production of IL8 as measured by ELISA (data not shown).
Figure 3 Urocortin II (UcnII) stimulates IL8 expression in human colonic epithelial cells via the nuclear factor‐κB (NF‐κB)‐ and mitogen activated protein (MAP) kinase‐dependent pathways. (A) ΙκBα, phospho p65 and phospho ERK (extracellularly regulated kinase) 1/2 expression in NCM460/CRHR2α cells treated with UcnII (10−7 M) for the indicated times as determined by western blot analysis. (B) Interleukin (IL)8 promoter transcription activity in NCM460/CRHR2α cells transfected with wild‐type IL8 promoter (IL8 WT), IL8 promoter with a mutated NF‐κB‐binding site (IL8 NF‐κBmut) and vector (PGL3 basic) after stimulation with UcnII (10−7 M) with or without astressin 2B for 6 h (*p<0.05, **p<0.01). (C) IL8 concentration (ng/ml) was determined using a human IL8 ELISA assay in NCM460/CRHR2α cells stimulated with UcnII (10−7 M) for 6 h with or without astressin 2B or the ERK1/2 antagonist PD98059 (*p<0.05).
Although both the NF‐κB and MAP kinase pathways have been associated with IL8 production in NCM460 colonocytes,19 whether they are required for UcnII‐associated IL8 expression is not known. To address this, we transfected CRHR2α‐expressing NCM460 cells with both wild‐type and mutant IL8 promoter plasmids with mutated NF‐κB‐binding sites as previously described.19,34 Cells were then stimulated with UcnII with or without the specific CRHR2 antagonist astressin 2B. Stimulation of cells containing wild‐type IL8 promoter with UcnII increased IL8 promoter activity, and this response was diminished by astressin 2B, suggesting CRHR2‐dependent activation of IL8 transcription (p<0.05, fig 3B). Astressin 2B did not prevent the TNFα‐induced increase in IL8 expression (data not shown), indicating the specificity of the UcnII response. Furthermore, the UcnII‐stimulated increase in IL8 transcription did not occur in cells transfected with the IL8 promoter containing a mutated NF‐κB‐binding site, providing evidence that NF‐κB is required for the induction of IL8 promoter activity by UcnII (fig 3B).
UcnII also stimulated increased IL8 secretion measured by ELISA in CRHR2α‐transfected cells, an effect that was significantly inhibited by astressin 2B (fig 3C). Since we previously reported that IL8 expression depends on ERK 1/2 activation,35 we evaluated the participation of ERK 1/2 in UcnII‐mediated IL8 secretion. We found that administration of the MEK1/2‐specific inhibitor PD98059 substantially reduced IL8 secretion in response to UcnII (p<0.05, fig 3C). Taken together, these results suggest that coupling of CRHR2α with UcnII stimulates IL8 production via activation of NF‐κB and MAP kinase pathways.
UcnII and CRHR2 are increased in human colitis
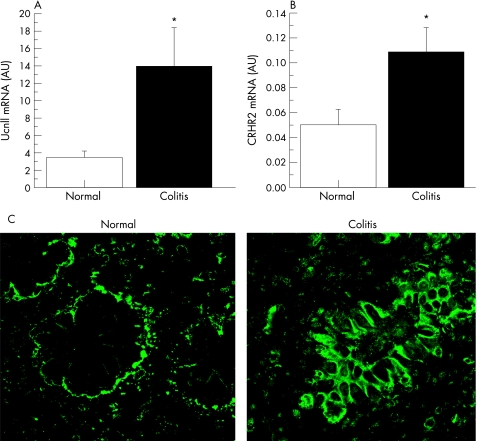
In light of this evidence for a pro‐inflammatory effect of CRHR2 and UcnII in colonocytes, we sought to determine if this ligand–receptor system is activated in patients with inflammatory bowel disease. Paired biopsies from both normal and inflamed mucosa from patients with ulcerative colitis and Crohn's colitis were obtained by colonoscopy. Our results showed increased expression by real‐time RT‐PCR of both UcnII and CRHR2 mRNA in patients with active colitis (p<0.05, fig 4A). In addition, immunofluorescence microscopy using an anti‐CRHR2 antibody demonstrated increased expression of CRHR2 in the colonic mucosa of patients with active colitis, with CRHR2 localised primarily on colonic epithelial cells and, to a lesser degree, in subepithelial tissues (fig 4B). Negative controls (see the Materials and methods section) showed no specific CRHR2 staining (data not shown).
Figure 4 Urocortin II (UcnII) and corticotropin‐releasing hormone receptor 2 (CRHR2) expression are increased in human colitis. (A) UcnII mRNA levels measured using real‐time PCR in mucosal samples from involved and non‐involved colonic mucosa areas from irritable bowel disease (IBD) subjects (expression levels normalised to TATA‐binding protein (TBP) expression across all samples, n = 8 per group, *p<0.05). (B) CRHR2 mRNA levels measured using real‐time PCR in mucosal samples from involved and non‐involved colonic mucosa areas from IBD subjects (expression levels normalised to TBP expression across all samples, n = 8 per group, *p<0.05). (C) Immunofluorescence of areas of normal mucosa (left) and active colitis (right) in patients with active IBD stained for antibody to CRHR2. Images are representative of three separate samples, each with similar findings.
Increased expression of CRHR2 and UcnII in human intestinal xenografts following C difficile toxin A stimulation
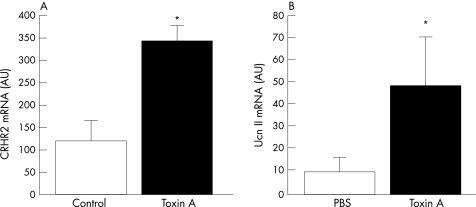
To confirm the presence of the CRHR2 receptor and its modulation by toxin A in an in vivo “human intestinal model”, we examined the effect of toxin A on CRHR2 expression in laser‐captured epithelial cells of human intestinal xenografts.16 We found that CRHR2 mRNA expression was significantly upregulated by toxin A treatment (by 2.5‐fold, fig 5A). To confirm whether UcnII is also expressed in human intestine in this model, we also determined UcnII mRNA levels in whole intestinal sections from toxin A‐exposed xenografts. Our results demonstrate that UcnII mRNA was expressed in the human colonic xenografts and was significantly upregulated 6 h after toxin A exposure (fig 5B).
Figure 5 (A) Corticotropin‐releasing hormone receptor 2 (CRHR2) mRNA levels measured using real‐time PCR in laser‐captured epithelial cells from colonic samples of toxin A‐ (10 μg) or buffer‐exposed human xenografts (expression levels normalised to glyceraldehyde phosphate dehydrogenase (GAPDH) expression across all samples, n = 4/group, *p<0.05). (B) Urocortin II (UcnII) mRNA levels measured using real‐time PCR in intestinal tissues of toxin A‐ (10 μg) or phosphate‐buffered saline (PBS)‐treated human xenografts (expression levels normalised to GAPDH expression across all samples, n = 4/group, *p<0.05).
Discussion
We show that CRHR2 expression is increased in human colonocytes in response to potent pro‐inflammatory stimuli such as TNFα and C difficile toxin A, and that this effect is prevented by pharmacological antagonism of NF‐κB (fig 1). These in vitro observations were confirmed by experiments in human intestinal xenografts in vivo demonstrating that toxin A can stimulate expression of human CRHR2 in colonic epithelial cells (fig 5). Importantly, we found increased expression of both UcnII and CRHR2 mRNA in patients with active IBD, and increased expression of CRHR2 protein in the colonic mucosa, most evident on colonic epithelial cells (fig 4). UcnII was also significantly upregulated in the mucosa of human colonic xenografts by toxin A (fig 5), indicating that it may represent an important CRHR2 ligand involved in the pathophysiology of intestinal inflammation. In support of this notion, exposure of human colonocytes to UcnII led to increased expression of the potent chemoattractant IL8 via NF‐κB‐ and ERK 1/2‐mediated pathways (fig 3). To our knowledge, this is the first evidence demonstrating that CRHR2 is linked to inflammatory responses in the human intestine, and extends our previous observations with CRHR2 knockout (KO) mice in terms of the importance of this receptor in intestinal inflammation.13
Previous studies showed that CRHR2 is present in both mouse and rat intestinal mucosa.36,37 CRHR2 mRNA and protein were expressed at low levels in the mouse ileum and dramatically upregulated during intestinal inflammation in response to C difficile toxin A.15 Our previous data demonstrated that CRHR2 KO mice exhibit a reduced intestinal inflammatory response to toxin A, and that pharmacological inhibition of CRHR2 has a similar effect.13 Here we report little CRHR2 mRNA expression in normal human colonic mucosa and cultured colonocytes, and increased expression of this receptor in toxin A‐exposed human colonic epithelial cells isolated from human intestinal xenografts, as well as in HT‐29 cells exposed to toxin A. The mechanism of toxin A‐induced increased expression of CRHR2 in our experiments has not been elucidated, but it might involve, at least in part, the transcription factor NF‐κB. Toxin A induces NF‐κB activation and stimulates expression of NF‐κB‐dependent pro‐inflammatory cytokines in mouse intestine32 and colonic epithelial HT‐29 cells.30 The fact that the potent NF‐κB inducer TNFα elicits this response further suggests that NF‐κB might be an important regulator of CRHR2 expression during an inflammatory response. In support of this notion, exposure of colonic epithelial HT‐29 cells to the proteasome inhibitor MG 132 inhibited toxin A‐induced CRHR2 expression. Moreover, promoter analysis of the CRHR2α human gene revealed the presence of a putative NF‐κB site at position –1036.10 CRHR2 expression is also positively regulated by glucocorticoids since there are several glucocorticoid‐responsive elements in the CRHR2α promoter.38 Interestingly, glucocorticoid levels are elevated during toxin A enteritis,39 along with increased CRHR2 expression.15 Clearly, detailed studies are needed to establish the importance of NF‐κB, other transcription factors or glucocorticoids in the modulation of CRHR2 gene expression during intestinal inflammation.
Consistent with our evidence, CRHR2 expression was also stimulated by IL1 and lipopolysaccharide in human mast cells.40 In contrast, IL1β reduced CRHR2 mRNA expression in rat vascular smooth muscle cells.41 Different cell phenotypes or different levels of CRHR2 expression might account for the different CRHR2s among different studies.
Our results demonstrating increased UcnII and CRH2R in the colonic mucosa of IBD patients, together with the pro‐inflammatory responses of cultured colonocytes exposed to UcnII, implicate CRHR2 and UcnII in IBD pathophysiology. Along these lines, Saruta et al found an increase in UcnI‐positive cells in the colonic mucosa of ulcerative colitis patients that was correlated with the severity of colitis,42 while Kawahito et al showed a slight increase in immunoreactive CRH in the colon of ulcerative colitis patients.43 Together with our findings, these results strongly suggest that the CRH family of peptides, as well as CRHR2, may participate in the control of colonic inflammation in IBD patients.
Our evidence indicates that both the NF‐κB and MAP kinase pathways are implicated in increased IL8 expression in response to UcnII. These results are similar to the results of Brar et al44 who showed that UcnII stimulated ERK 1/2 phosphorylation in CRHR2‐expressing cells. UcnII‐induced vasodilatation in rat thoracic aorta also involves p38 MAP kinase as well as protein kinase A pathways.14 In contrast, UcnII enhances IL10, and decreases TNFα release in murine RAW264.7 macrophages,8 indicating that UcnII may also elicit anti‐inflammatory responses. Gonzalez‐Rey et al recently reported that peripherally administered UcnI, a ligand for CRHR1 and CRHR2, ameliorates trinitrobenzenesulphonic acid (TNBS)‐induced colitis, although the mechanistic actions of this effect are unclear.45
The ability of UcnII to activate NF‐κB directly and degrade its inhibitor IκB via CRHR2 signalling has not previously been recognised. However, the UcnII‐related peptide CRH has been shown to stimulate NF‐κB DNA‐binding activity with parallel degradation of its inhibitor in both thymocytes and keratinocytes.46,47
In summary, our findings support an important pro‐inflammatory role for intestinal UcnII and CRHR2 in both acute and chronic human colitis. Our results also provide a novel mechanistic paradigm, involving modulation of CRHR2 by a bacterial exotoxin at the colonocyte level, and increased expression of its ligand, UcnII, from intestinal mucosal cells in response to the same enterotoxin. Results indicating that the CRH family of peptides and their receptors participate in the pathophysiology of both stress‐induced intestinal responses48 and, as shown previously15,49 and here, in human intestinal inflammation, point to an important role for these molecules in the pathway(s) by which brain–gut interactions may modulate inflammatory responses. Together with our previous findings,13,15,49 results from this current study point to a possible application of CRHR2 antagonists in the treatment of intestinal inflammation of various aetiologies.
Acknowledgements
We are grateful to the staff of the GI Endoscopy Unit of Beth Israel Deaconess Medical Center and especially to Eoin Kelly for assistance with the collection of human samples. We would like also to thank Rachel Diamond and Charles Vanderburg at the Advanced Tissue Resource Center, Harvard Center for Neurodegeneration and Repair for their excellent work in laser capture microscopy, and Justine Curley in the Histology Core of Beth Israel Deaconess Medical Center for help with the immunoflourescence studies. The IL8 promoter–luciferase constructs were a kind gift from Dr Andrew Keates, Division of Gastroenterology, Beth Israel Deaconess Medical Center.
Abbreviations
CRH - corticotropin‐releasing hormone
CRHR2 corticotropin‐releasing hormone receptor 2 -
DMEM - Dulbecco's modified Eagle's medium
DMSO - dimethylsulphoxide
DTT - dithiothreitol
ERK - extracellularly regulated kinase
FITC - fluorescein isothiocyanate
GAPDH - glyceraldehyde phosphate dehydrogenase
HUVEC - human umbilical vein epithelial cell
IBD - inflammatory bowel disease
IL - interleukin
KO - knockout
LCM - laser capture microdissection
MAP - mitogen‐activated protein
MCP - monocyte chemoattractant protein
NF‐κB - nerve factor‐κB
PBS - phosphate‐buffered saline
RTR‐PCR - reverse transcription‐PCR
TBP - TATA‐binding protein
TBS - Tris‐buffered saline
TNBS - trinitrobenzenesulphonic acid
TNF - tumour necrosis factor
Ucn - urocortin
Footnotes
Funding: Supported by research grant PO‐1 DK 33506 from the National Institutes of Health (C Pothoulakis), and the Crohn's and Colitis Foundation (T Savidge). A C Moss is the recipient of the AGA/Centocor International Research Fellowship in Intestinal Inflammation & Immunity 2005.
Competing interests: None.
References
- 1.Grigoriadis D E, Lovenberg T W, Chalmers D T.et al Characterization of corticotropin‐releasing factor receptor subtypes. Ann N Y Acad Sci 199678060–80. [DOI] [PubMed] [Google Scholar]
- 2.Bamberger C M, Bamberger A M. The peripheral CRH/urocortin system. Ann N Y Acad Sci 2000917290–296. [DOI] [PubMed] [Google Scholar]
- 3.Hsu S Y H A. Human stresscopin and stresscopin‐related peptide are selective ligands for the type 2 corticotropin‐releasing hormone receptor. Nat Med 20017605–611. [DOI] [PubMed] [Google Scholar]
- 4.Reyes T M, Lewis K, Perrin M H.et al Urocortin II: a member of the corticotropin‐releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 2001982843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.la Fleur S E, Wick E C, Idumalla P S.et al Role of peripheral corticotropin‐releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci USA 20051027647–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen A B A, Vaughan J, Brar B.et al Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin‐releasing factor receptor (CRFR) 1‐ and CRFR2‐null mice, and regulation by glucocorticoids. Endocrinology 20041452445–2457. [DOI] [PubMed] [Google Scholar]
- 7.Martinez V W L, Rivier J E, Vale W.et al Differential actions of peripheral corticotropin‐releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 2002301611–617. [DOI] [PubMed] [Google Scholar]
- 8.Sashinami H, Kageyama K, Suda T.et al Urocortin 2 suppresses host resistance to Listeria monocytogenes infection via up‐regulation of interleukin‐10. Endocrinology 20051465003–5011. [DOI] [PubMed] [Google Scholar]
- 9.Tsatsanis C, Androulidaki A, Dermitzaki E.et al Urocortin 1 and urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett 20055794259–4264. [DOI] [PubMed] [Google Scholar]
- 10.Catalano R D, Kyriakou T, Chen J.et al Regulation of corticotropin‐releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol 200317395–410. [DOI] [PubMed] [Google Scholar]
- 11.Kostich W A, Chen A, Sperle K.et al Molecular identification and analysis of a novel human corticotropin‐releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol 1998121077–1085. [DOI] [PubMed] [Google Scholar]
- 12.Valdenaire O, Giller T, Breu V.et al A new functional isoform of the human CRF2 receptor for corticotropin‐releasing factor. Biochim Biophys Acta 19971352129–132. [DOI] [PubMed] [Google Scholar]
- 13.Kokkotou E, Torres A. Moss AC, et al. Corticotropin‐releasing hormone receptor 2 deficient mice have reduced intestinal inflammatory responses. J Immnol 20061773355–3361. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama K, Furukawa K, Miki I.et al Vasodilative effects of urocortin II via protein kinase A and a mitogen‐activated protein kinase in rat thoracic aorta. J Cardiovasc Pharmacol 200342561–565. [DOI] [PubMed] [Google Scholar]
- 15.Wlk M, Wang C C, Venihaki M.et al Corticotropin‐releasing hormone antagonists possess anti‐inflammatory effects in the mouse ileum. Gastroenterology 2002123505–515. [DOI] [PubMed] [Google Scholar]
- 16.Savidge T C, Pan W H, Newman P.et al Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 2003125413–420. [DOI] [PubMed] [Google Scholar]
- 17.Rivier J, Gulyas J, Kirby D.et al Potent and long‐acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem 2002454737–4747. [DOI] [PubMed] [Google Scholar]
- 18.Rivier C L, Grigoriadis D E, Rivier J E. Role of corticotropin‐releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology 20031442396–2403. [DOI] [PubMed] [Google Scholar]
- 19.Zhao D, Keates A C, Kuhnt‐Moore S.et al Signal transduction pathways mediating neurotensin‐stimulated interleukin‐8 expression in human colonocytes. J Biol Chem 200127644464–44471. [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Cetrulo C L, Theoharides T C. Corticotropin‐releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen‐activated protein kinase pathway. Mol Pharmacol 200669998–1006. [DOI] [PubMed] [Google Scholar]
- 21.Darmoul D, Gratio V, Devaud H.et al Aberrant expression and activation of the thrombin receptor protease‐activated receptor‐1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol 20031621503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawhney R S, Sharma B, Humphrey L E.et al Integrin alpha2 and extracellular signal‐regulated kinase are functionally linked in highly malignant autocrine transforming growth factor‐alpha‐driven colon cancer cells. J Biol Chem 200327819861–19869. [DOI] [PubMed] [Google Scholar]
- 23.Jobin C, Hellerbrand C, Licato L L.et al Mediation by NF‐kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM‐1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut 199842779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palombella V J, Rando O J, Goldberg A L.et al The ubiquitin–proteasome pathway is required for processing the NF‐kappa B1 precursor protein and the activation of NF‐kappa B. Cell 199478773–785. [DOI] [PubMed] [Google Scholar]
- 25.Scherer D C, Brockman J A, Chen Z.et al Signal‐induced degradation of I kappa B alpha requires site‐specific ubiquitination. Proc Natl Acad Sci USA 19959211259–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walmsley R S, Ayres R C, Pounder R E.et al A simple clinical colitis activity index. Gut 19984329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Best W R, Becktel J M, Singleton J W.et al Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 197670439–444. [PubMed] [Google Scholar]
- 28.Castagliuolo I, Riegler M, Pasha A.et al Neurokinin‐1 (NK‐1) receptor is required in Clostridium difficile‐induced enteritis. J Clin Invest 19981011547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoncini T, Apa R, Reis F M.et al Human umbilical vein endothelial cells: a new source and potential target for corticotropin‐releasing factor. J Clin Endocrinol Metab 1999842802–2806. [DOI] [PubMed] [Google Scholar]
- 30.He D, Sougioultzis S, Hagen S.et al Clostridium difficile toxin A triggers human colonocyte IL‐8 release via mitochondrial oxygen radical generation. Gastroenterology 20021221048–1057. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson K K, Smith M F, Jr, Bobak D A. Roles of intracellular calcium and NF‐kappa B in the Clostridium difficile toxin A‐induced up‐regulation and secretion of IL‐8 from human monocytes. J Immunol 19991635183–5191. [PubMed] [Google Scholar]
- 32.Warny M, Keates A C, Keates S.et al p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL‐8 production, and enteritis. J Clin Invest 20001051147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyer M P, Manzano L A, Merriman R L.et al NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim 199632315–317. [DOI] [PubMed] [Google Scholar]
- 34.Karagiannides I, Kokkotou E, Tansky M.et al Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA 20061035207–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee S H, Keates A C, Moyer M P.et al MEK is a key modulator for TLR5‐induced interleukin‐8 and MIP3alpha gene expression in non‐transformed human colonic epithelial cells. J Biol Chem 200427925179–25188. [DOI] [PubMed] [Google Scholar]
- 36.Perrin M, Donaldson C, Chen R.et al Identification of a second corticotropin‐releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA 1995922969–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatzaki E, Crowe P D, Wang L.et al CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem 200490309–316. [DOI] [PubMed] [Google Scholar]
- 38.Chen A, Perrin M, Brar B.et al Mouse corticotropin‐releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol 200519441–458. [DOI] [PubMed] [Google Scholar]
- 39.Castagliuolo I, Karalis K, Valenick L.et al Endogenous corticosteroids modulate Clostridium difficile toxin A‐induced enteritis in rats. Am J Physiol Gastrointest Liver Physiol 2001280G539–G545. [DOI] [PubMed] [Google Scholar]
- 40.Papadopoulou N G, Oleson L, Kempuraj D.et al Regulation of corticotropin‐releasing hormone receptor‐2 expression in human cord blood‐derived cultured mast cells. J Mol Endocrinol 200535R1–R8. [DOI] [PubMed] [Google Scholar]
- 41.Kageyama K, Suda T. Regulation of corticotropin‐releasing factor receptor type 2beta messenger ribonucleic acid by interleukin‐1beta in rat vascular smooth muscle cells. Neuroimmunomodulation 20019326–332. [DOI] [PubMed] [Google Scholar]
- 42.Saruta M, Takahashi K, Suzuki T.et al Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab 2004895352–5361. [DOI] [PubMed] [Google Scholar]
- 43.Kawahito Y, Sano H, Mukai S.et al Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut 199537544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brar B K, Chen A, Perrin M H.et al Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin‐releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology 20041451718–1729. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez‐Rey E, Fernandez‐Martin A, Chorny A.et al Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn's disease. Gut 200655824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao J, Karalis K P. Regulation of nuclear factor‐kappaB by corticotropin‐releasing hormone in mouse thymocytes. Mol Endocrinol 2002162561–2570. [DOI] [PubMed] [Google Scholar]
- 47.Zbytek B, Pfeffer L M, Slominski A T. Corticotropin‐releasing hormone stimulates NF‐kappaB in human epidermal keratinocytes. J Endocrinol 2004181R1–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tache Y, Perdue M H. Role of peripheral CRF signalling pathways in stress‐related alterations of gut motility and mucosal function. Neurogastroenterol Motil 200416(Suppl 1)137–142. [DOI] [PubMed] [Google Scholar]
- 49.Anton P M, Gay J, Mykoniatis A.et al Corticotropin‐releasing hormone (CRH) requirement in Clostridium difficile toxin A‐mediated intestinal inflammation. Proc Natl Acad Sci USA 20041018503–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]