Abstract
Immunocompetence (i.e., the ability to produce an immune response to pathogens) can be predicted to influence the chances that organisms have to survive and reproduce. In this study we simulated a challenge to the immune systems of male barn swallows (Hirundo rustica) by injecting them intraperitoneally with a multigenic antigen, sheep red blood cells, and we analyzed long-term survival in relation to their immunocompetence. Males were assigned to four groups that differed for the treatment of the length of the outermost tail feathers, a sexually dimorphic ornamental character that is currently under directional sexual selection. Immunocompetence was measured as change of concentration of gamma globulins relative to plasma proteins. The intensity of the immune response was independent of age. Males that showed the highest short-term response to sheep red blood cells were more likely to survive until the breeding season following that in which they had been inoculated, a pattern consistently observed within each experimental group. Males with comparatively long tails were more likely to survive than those with short tails. To our knowledge, the results of this study are the first to demonstrate that immunocompetence can predict long-term survival in a free-ranging vertebrate. Moreover, they are compatible with current models of parasite-mediated sexual selection because long-tailed males are more immunocompetent than short-tailed ones, and females, by preferring to mate with the most ornamented males, may acquire the “good genes” for high immunocompetence and, hence, for high viability of their offspring.
Keywords: gamma globulins, ornaments/sexual selection/sheep red blood cells/survival
Birds, like most other animals, live in complex environments in which pathogenic organisms are abundant. Many of these organisms, from viruses to parasitic metazoans, exert a negative effect on the fitness of the individuals they infect (1, 2). The ability to produce an immune response to pathogens can therefore be predicted to play a fundamental role in determining the chances that individuals have to survive and reproduce. Vertebrates have evolved the ability to produce sophisticated immune responses that usually involve both cellular and humoral mechanisms. In birds in particular, cellular immunity is typically mediated by heterophilic granulocytes and lymphocytes (3–6). Gamma globulins include a large family of plasma proteins, such as immunoglobulins, that are the source of antibodies involved in humoral immune responses. In captive birds, experimental infection with a variety of antigenic agents has resulted in the increase of immunoglobulins in various tissues, including blood (7–10).
Although the above information seems to suggest the existence of a causal link between immunocompetence (i.e., the ability to produce an adaptive immune response) and survival, to our knowledge no data have been published on this topic for free-living animals.
The aim of our study was to analyze the relationship between immunocompetence and long-term survival of male barn swallows (Hirundo rustica) in which an immune response has been elicited by intraperitoneal inoculation of a suspension of sheep red blood cells (SRBCs) (11, 12). Our simple, specific prediction was that males with a high immune response to SRBCs during a breeding season were more likely to survive until the following breeding season than males with a weak response.
SRBC inoculation is a standard procedure for immunocompetence measures (11, 12). Importantly, in poultry it has been shown that lines selected for high antibody responses to this multigenic antigen are more resistant to various infectious diseases (13, 14). Hence, individuals that are more responsive to SRBCs may exhibit a higher level of immune-mediated general disease resistance.
Previous studies on birds have shown that SRBC inoculation results in an increase of immunoglobulin plasma levels (12). In a previous study on the barn swallow (15), SRBC inoculation resulted in an increase in gamma globulin levels relative to the total content of proteins in the plasma. However, no effect was observed on either relative counts of five different types of leukocytes (heterophils, basophils, eosinophils, lymphocytes, and monocytes) or their concentration relative to red blood cells (15). In the present paper, we will therefore deal only with the relationship between survival and change of gamma globulin levels following SRBC treatment. We also showed that experimental increase of the expression of a male ornamental character (length of the outermost tail feathers) negatively influenced the intensity of antibody response, and we interpreted this as direct evidence of immune response being costly.
The barn swallow is a socially monogamous, colonial, migratory passerine whose biology and ecology have been intensively studied (16). Relevant to this study are the facts that (i) males are affected by a variety of endo- and ectoparasites, some of which are known to depress the fitness of the host (17), and (ii) females prefer to mate with males with long tail ornaments (16, 18).
STUDY AREA AND METHODS
The study was carried out during spring 1994 and 1995 on six farms located east of Milan (northern Italy). In spring 1994, male barn swallows were caught in mist nets at the time of their arrival at the colonies. Each individual was marked with a metal ring on one leg and a plastic, colored ring on the other. Individuals were sexed according to the shape of the cloacal protuberance (19), and this was later confirmed by inspection for presence (female) or absence (male) of an incubation patch and by observation of sexual and breeding behavior. At the time of first capture we measured several morphological variables, including length of the left and right outermost tail feathers, as well as several parasitological and hematological variables (15). Tail length was expressed as the mean length of the two outermost tail feathers. Blood samples (on average, 200 μl in heparinized hematocrit capillary tubes) and smears were also taken at first capture and at recapture(s). Blood samples were then centrifuged for 10 min at 4000 rpm, and plasma was stored at −30°C for gamma globulin analysis.
We assigned males sequentially to one of four experimental groups (i.e., the first, second, third, and fourth males to be captured were assigned to the first, second, third, and fourth groups, respectively, and so on for the next males). All males were injected intraperitoneally with 100 μl of a PBS solution containing 5 × 105 SRBCs per μl of solution. Males of the first group were given the above treatment and released. Both outermost tail feathers of males of the second group were cut at ≈1 cm from the base and then reglued. Males of the third group had their outermost tail feathers shortened by 2 cm by cutting them at ≈1 cm from the base of the feather, and males of the fourth group had their tails elongated by 2 cm.
We checked for unbiased assignment of males to the four experimental groups by comparing the values of all the morphological, parasitological, and hematological variables measured at the time of first capture in 1994. None of these variables showed a significant difference among the groups (one-way ANOVA, F values always associated with P-values larger than 0.05; see also ref. 15). Although this result is not relevant to the main aim of the present study, which was to analyze differences in immunocompetence between survivors and nonsurvivors within each of the tail treatment groups, it shows that our procedure of assignment did not produce any biases in the composition of the groups; hence, survival data can also be analyzed in relation to tail treatment.
In spring 1995 we captured the barn swallows used in our study colonies by the same general procedures used the previous year. We attempted to catch as many individuals as possible during frequent capture sessions (8–11 capture sessions lasting several hours in each farm from late March to late June). The fact that almost all (147 out of 150; 98%) of the birds captured in the last capture session in each farm had already been captured during the same breeding season clearly suggests that very few birds escaped our nets. In our study area, male barn swallows show extreme fidelity to the breeding site. Male swallows, including those considered in the present study, were never recorded to have moved to another farm in year i + 1 after having bred in year i in the nearest neighboring farm (4 pairs of nearest neighboring colonies, 3 breeding seasons, n > 150 males in each breeding season) or in any other farm in our study area (15 or more farms in each breeding season, range of distances between farms: 0.4–20 km, 3 breeding seasons, n > 300 males in each breeding season; N.S. and A.P.M., unpublished data). In each year and farm, some of the males did not succeed in acquiring a mate. Furthermore, none of these males was found to have moved to another farm. We therefore assumed that birds that were inoculated in 1994 and were not recaptured in 1995 had not survived.
Because we also performed an intensive capture effort during spring 1993, in spring 1994 we could classify males that were not ringed or were ringed as nestlings during the previous breeding season as birds born in spring 1993, and males that were already ringed as adults during 1993 as birds that were 2 years old or older.
For the purposes of this paper, we define survivors as males that were recaptured in the second study year (1995), and nonsurvivors as males that were not recaptured in 1995.
Gamma globulins were assayed, on average, 3 months after blood collection by densitometric analysis after electrophoretic separation of plasma proteins on agarose gels (Paragon SPE Kit, Beckman). Plasma (5 μl) was diluted 1:2.5 in Barbital buffer (pH 8.6). The diluted sample (5 μl) was applied to agarose gels after standard procedures with the Paragon SPE kit. The electrophoreses were applied at constant voltage (100 V) at 20°C for 25 min. After electrophoresis, gels were air-dried and stained following the manufacturer’s instructions. Densitometric analysis was performed by a computer image analysis procedure run by the gelanalyst program (Eidosoft, Somma Lombardo, Italy). The relative concentration of gamma globulins and other proteins that comigrate during electrophoresis was expressed as the ratio between the area of the densitometric profile corresponding to the gamma globulin region and the total area of the densitometric profile. Change of relative gamma globulin levels between the capture in which we inoculated SRBCs and recapture was expressed as the difference between relative levels recorded in the blood samples collected at the time of recapture and that recorded at the time of first capture (see refs. 15 and 20 for further details on assay procedures and repeatability of within-sample relative gamma globulin levels). Time elapsed from the capture in which males were inoculated, and recapture did not differ among experimental groups (F3,81 = 0.34, not significant; mean ± SE intercapture time, in days, was 24.9 ± 4.33 for unmanipulated males, 19.4 ± 3.83 for males whose tail was cut and reglued, 20.9 ± 3.30 for tail-shortened males, and 22.8 ± 4.03 for tail-elongated males).
RESULTS
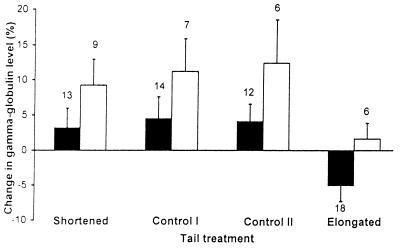
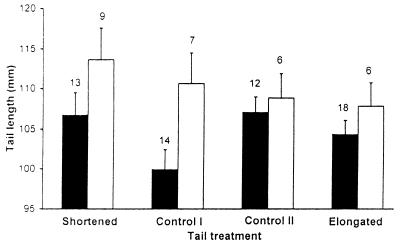
We predicted that survivors should have been the most immunocompetent males (i.e., males that had comparatively large changes in gamma globulin levels). Moreover, we expected males with naturally large tail ornaments to be more likely to survive than those with short ones, because a positive relationship between ornament size and survival has been demonstrated previously (16). The results clearly confirmed our predictions. Mean change in gamma globulins and mean premanipulation tail length of survivors were larger than those of nonsurvivors. These differences between survivors and nonsurvivors were consistently found in all the experimental groups (Figs. 1 and 2). However, we could show no clear pattern for the effect of age on survival. We tested for the simultaneous, independent effects of immunocompetence, premanipulation tail length, and age on survival in a logistic regression analysis in which these variables were entered as regressors and survival was entered as the response variable (Table 1). To simultaneously test for the effect of tail treatment we included in the regression model three dummy variables specifying pairwise comparisons between survival of males that did not have their tails manipulated and males that had their tails cut and reglued, shortened, or elongated, respectively. The effects of change of gamma globulins and premanipulation tail length were statistically highly significant, thus indicating that immunocompetence significantly contributed to predicting survival even after controlling for the independent effect of tail length. The effect of age, however, did not achieve significance. None of the pairwise comparisons between survival of unmanipulated males and survival of those in the other groups showed a statistically significant difference when the other regressors were taken into account. However, males with shortened tails had much higher chances of surviving than males whose tails were experimentally elongated (survivors/total number of males: shortened, 0.41, n = 22; and elongated, 0.25, n = 24), whereas survival of males of the two control groups was identical (0.33; unmanipulated males, n = 21, and males whose tails were cut and reglued, n = 18) and intermediate between that of males whose tail length was altered. When we included first-degree interaction terms between predictor variables in the logistic regression, none significantly increased the variance explained by the model.
Figure 1.
Mean (+SE) change of gamma globulin levels at approximately 3 weeks from inoculation of SRBCs of male swallows whose tails were unmanipulated (Control I), cut and reglued (Control II), and shortened or elongated by 20 mm, that survived (white bars) and did not survive (black bars) until the breeding season following that of inoculation. Measurement unit of gamma globulins is the percentage of gamma globulins relative to whole protein plasma content. Change is expressed as difference between value at recapture and value at first capture. Numbers are sample sizes.
Figure 2.
Mean (+SE) premanipulation tail length of male swallows whose tails were unmanipulated (Control I), cut and reglued (Control II), and shortened or elongated by 20 mm, that survived (white bars) and did not survive (black bars) until the breeding season following that of inoculation.
Table 1.
Results of a logistic regression analysis in which the response variable was survival, entered as a two-state variable; change in gamma globulin level, premanipulation tail length, and age were the independent regressors; and three dummy variables accounted for the pairwise comparisons between the group of males whose tails were not manipulated (Control I) and the groups of males whose tails were cut and reglued (Control II), shortened, or elongated, respectively.
| Predictor | χ2 | P |
|---|---|---|
| Control I vs. Control II | 0.22 | NS |
| Control I vs. shortened | 0.00 | NS |
| Control I vs. elongated | 0.01 | NS |
| Change of gamma globulins | 7.59 | 0.006 |
| Premanipulation tail length | 7.30 | 0.007 |
| Age | 0.04 | NS |
NS, not significant.
DISCUSSION
Our results clearly support the idea that long-term survival rates of male barn swallows are higher for birds that are more responsive to an experimental challenge to their immune system or that have large tail ornaments, after controlling for the effect of covariates. We scrutinized our experimental procedures to identify potential biases in the results. Males assigned to the experimental groups, on average, did not differ in any of the morphological, parasitological, or hematological variables measured in 1994 (15). Our recapture data indicate that very few swallows escaped our capture attempts, and emigration of breeding males from one colony to another in the following breeding season, if it occurs, is a rare event. Indeed, in a sample of more than 1000 males during three breeding seasons, none is known to have moved to another farm to breed. Although we cannot rule out the possibility that some males remained uncaptured in 1995 or emigrated, this would have produced the appearance of a larger immunocompetence of “survivors” than “nonsurvivors” only if males with smaller immunocompetence were less likely to be captured or were more likely to emigrate. However, we see no reasons for speculating that such relationships between immunocompetence and breeding site fidelity or catchability of males exist, and we consider these possibilities very remote.
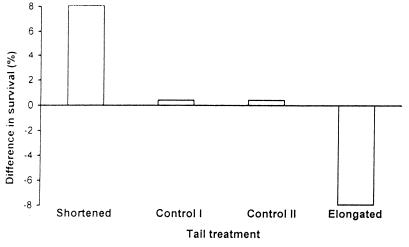
The results presented in this paper about the effect of tail length manipulation are in line with those obtained in four previous independent experiments, both in terms of quality and of intensity of the effect (16). Similar to previous findings, males with experimentally elongated tails suffered an increase in mortality of approximately 8% compared with the overall estimate of survival for all males included in the four experimental groups, whereas survival of tail-shortened males increased by approximately the same magnitude (Fig. 3). This evidence has been interpreted as the result of impaired foraging ability and/or increased aerodynamic costs of flight. The positive relationship between natural tail length and survival also is not a novel one and has been described for a large sample of male swallows from Denmark (16).
Figure 3.
Difference in survival between male swallows whose tails were unmanipulated (Control I), cut and reglued (Control II), and shortened or elongated by 20 mm. Zero represents the survival (survivors/total number of males) of males of all the groups pooled.
The significance of our study mainly lies in the experimental evidence provided for a positive relationship between immunocompetence and survival. To our knowledge, this is the first study in which such a relationship has been demonstrated under field experimental conditions.
The importance of pathogens and parasites in animal life has been widely acknowledged and documented. Many studies have shown that immune response to pathogens, in birds and in other vertebrates, involves production of antibodies by a variety of cell types in different tissues. Therefore, there seems to be little doubt that, in most vertebrates, humoral immune response is central to the adaptive immunity processes. SRBCs are multigenic antigens that may cross-react with antibodies to other antigens previously experienced by the host and also eliciting an immune response. Although this may represent a limitation of using SRBCs, it is most important to the present study that selection for high antibody responses to SRBC antigens in other birds resulted in lines more resistant to various infectious diseases (13, 14). Hence, the positive association between immunocompetence and survival we observed may result from immunocompetent males being better able to cope with a variety of pathogens.
Saino and Møller (15) showed that for one experimental group of males considered in this paper (males with elongated tails), a positive correlation existed between premanipulation tail length and immunocompetence, and therefore a secondary sexual character currently subject to a directional female mate preference reflects the ability to produce immune responses. Moreover, in previous studies (16) and in the present one we have shown that males with large tail ornaments survive better than those with small ornaments. These pieces of evidence combined with the results presented here thus suggest that differential viability in relation to tail ornamentation may at least partly arise because males with large ornaments are more immunocompetent.
This study has obvious relevance to current models of sexual selection, particularly to those models that envisage sexual selection as mediated by female preference for parasite- and disease-resistant males. Selective advantages may arise from genetic resistance to pathogens (21–23) and efficient immune systems (24–26). Manipulative studies currently in progress will clarify the extent to which additive genetic variance contributes to the total variance in the intensity of immune response observed in this study. At present, we can speculate that if interindividual differences in immunocompetence have a genetic basis, then our results most likely would be the first to show that female mate preference for highly ornamented males will result in acquisition of “good genes” for high immunocompetence for their offspring. By mating with long-tailed males, female barn swallows may thus acquire genes for immunocompetence that ultimately will ensure high viability to their offspring. Hence, this study clarifies one of the mechanisms through which genetic benefits may accrue to choosy females from their mate preference.
Acknowledgments
We are grateful to M. Krivacek, A. Foti, and L. Savar for their assistance during field and laboratory work. This research has been supported by grants from the Italian Consiglio Nazionale delle Ricerche and the Danish Natural Science Research Council.
Note Added in Proof:
If survival is affected by immune response, then we would predict no relationship between tail length (independent variable) and immune response (dependent variable) after controlling for the effects of treatment, survival, and age (additional independent variables). Indeed, the effects of tail length and age were nonsignificant (tail length: F1,78 = 0.53, NS; age: F1,78 = 0.13, NS), whereas the effects of treatment and survival were significant (treatment: F3,78 = 4.48, P = 0.006; survival: F1,78 = 8.50, P = 0.005). This indicates that tail length reliably signals survival prospects mediated by immune defense.
Footnotes
Abbreviation: SRBC, sheep red blood cell.
References
- 1.Price P W. Evolutionary Biology of Parasites. Princeton: Princeton Univ. Press; 1980. [Google Scholar]
- 2.Lehmann T. Parasitol Today. 1993;9:8–13. doi: 10.1016/0169-4758(93)90153-7. [DOI] [PubMed] [Google Scholar]
- 3.Rose M E, Hesketh P, Ogilvie B M. Immunology. 1979;36:71–79. [PMC free article] [PubMed] [Google Scholar]
- 4.Davis P J. In: Avian Immunology. Rose M E, Payne L N, Freeman B M, editors. Edinburgh: British Poultry Science; 1981. pp. 361–385. [Google Scholar]
- 5.Hawkey C M, Samour J H, Ashton D G. Avian Pathol. 1983;12:73–84. doi: 10.1080/03079458308436150. [DOI] [PubMed] [Google Scholar]
- 6.Averbeck C. Avian Pathol. 1992;21:215–223. doi: 10.1080/03079459208418837. [DOI] [PubMed] [Google Scholar]
- 7.DeVaney J A, Augustine P C. Poult Sci. 1988;67:549–556. doi: 10.3382/ps.0670549. [DOI] [PubMed] [Google Scholar]
- 8.Parmentier H K, Siemonsma R, Nieuwland M G B. Poult Sci. 1994;73:825–835. doi: 10.3382/ps.0730825. [DOI] [PubMed] [Google Scholar]
- 9.Jayawardane G W L, Spradbrow P B. Vet Microbiol. 1995;46:69–77. doi: 10.1016/0378-1135(95)00073-j. [DOI] [PubMed] [Google Scholar]
- 10.Russell P H, Ezeifka G O. Vaccine. 1995;13:61–66. doi: 10.1016/0264-410x(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 11.Tsiagbe V K, Cook M E, Harper A E, Sunde M L. Poult Sci. 1987;66:1147–1154. doi: 10.3382/ps.0661147. [DOI] [PubMed] [Google Scholar]
- 12.Lochmiller R L, Vestrey M R, Boren J C. Auk. 1993;110:503–510. [Google Scholar]
- 13.Gross W G, Siegel P B, Hall W, Domermuth C H, DuBoise R T. Poult Sci. 1980;59:205–210. doi: 10.3382/ps.0590205. [DOI] [PubMed] [Google Scholar]
- 14.Dunnington E A, Martin A, Briles W E, Briles R W, Siegel P B. Arch Gefluegelkd. 1986;50:94–96. [Google Scholar]
- 15.Saino N, Møller A P. Behav Ecol. 1996;7:227–232. [Google Scholar]
- 16.Møller A P. Sexual Selection and the Barn Swallow. Oxford: Oxford Univ. Press; 1994. [Google Scholar]
- 17.Møller A P. In: Bird-Parasite Interactions: Ecology, Evolution and Behavior. Loye J, Zuk M, editors. Oxford: Oxford Univ. Press; 1991. pp. 328–348. [Google Scholar]
- 18.Møller A P. Nature (London) 1988;332:640–642. [Google Scholar]
- 19.Svensson L. Identification Guide to European Passerines. Stockholm: Naturhistoriska Riksmuseet; 1984. [Google Scholar]
- 20.Saino N, Møller A P, Bolzern A M. Behav Ecol. 1995;6:397–404. [Google Scholar]
- 21.Hamilton W D, Zuk M. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 22.Møller A P. J Evol Biol. 1990;3:319–328. [Google Scholar]
- 23.Clayton D H. Parasitol Today. 1991;7:329–334. doi: 10.1016/0169-4758(91)90211-6. [DOI] [PubMed] [Google Scholar]
- 24.Folstad A, Karter A J. Am Nat. 1992;139:603–622. [Google Scholar]
- 25.Møller A P, Saino N. J Parasitol. 1994;80:850–858. [PubMed] [Google Scholar]
- 26.Møller, A. P., Dufva, R. & Erritzøe, J. (1997) Evolution, in press.