Abstract
Background
Cigarette smoking (CS) promotes endothelial dysfunction and atherosclerosis in the vascular bed. The impact of smoking on atrial myocardium is not defined in humans.
Objective
To determine the effect of CS on the development of interstitial fibrosis in atrial myocardium.
Design
Case–control study.
Patients
95 patients (46 smokers and 49 non‐smokers) undergoing coronary artery bypass grafting (CABG).
Main outcome measures
Amount of atrial fibrosis, collagen I, III and IV expression pattern, and quantitative reverse transcriptase‐PCR. Occurrence of postoperative atrial fibrillation (AF).
Results
In the study population, patient age correlated significantly with the amount of atrial fibrosis (r = 0.18; p<0.05). Nicotine misuse (pack years) was identified as the only factor related to atrial fibrosis in smokers (r = 0.311; p<0.05). The amount of fibrosis was higher in patients with postoperative AF (22.9% (6.2%) vs. 27.0% (8.2%); p<0.05). To show a causal relationship between CS and atrial fibrosis, atrial tissue slices from non‐smokers (n = 8) were cultured in the presence of nicotine base (185 and 740 nmol/l). Nicotine base induced mRNA expression of collagen III (up to 10‐fold) in a concentration‐dependent manner resembling the immunohistological collagen expression pattern observed in CS.
Conclusion
CS contributes to the development of atrial fibrosis via nicotine. Atrial fibrosis by itself has been shown to provide an arrhythmogenic substrate, which may increase the likelihood of the occurrence of atrial arrhythmias, including postoperative AF.
Cigarette smoke is a complex mixture of chemicals that includes nicotine as well as toxic substances such as carbon monoxide and polycyclic aromatic hydrocarbons.1 Inhalation of these substances predisposes to several different atherosclerotic syndromes1,2,3,4,5 and is also associated with the occurrence of cardiac arrhythmia.6,7 However, the proarrhythmic effect of cigarette smoking (CS) seems to depend on the nicotine concentration in the blood.6 Increased nicotine levels raise atrial and ventricular vulnerability to fibrillation.6,7,8,9,10 These profibrillatory effects seem to depend on the inhibition of ion channels and conduction‐slowing properties.7,8 However, the structural and molecular basis by which prolonged exposure to nicotine might lead to atrial fibrillation (AF) has not been fully explored.7,8,9,10
Recent studies have clearly shown that atrial fibrosis provides a fixed morphological substrate that favours the occurrence of atrial arrhythmias in response to a triggering event.11,12,13,14 Interstitial fibrosis isolates groups of atrial myocytes as well as individual myocytes. It also impairs cell‐to‐cell coupling, causing inhomogeneities in intra‐ and interatrial conduction, which may impair atrial transport function and increase the likelihood of AF.11 Therefore, the elucidation of pathophysiological mechanisms leading to accumulation of collagen in the atria seems to be of clinical relevance. Nevertheless, the impact of CS on fibrotic changes in atrial myocardium has hitherto not been explored. In this study, we investigated the association between CS and the amount of atrial fibrosis in patients undergoing coronary artery bypass grafting (CABG). An organotypic tissue culture model was used to determine a causal relationship between nicotine and collagen expression in human atrial tissue samples.
Methods
Patient population
Right atrial appendages were obtained from 95 consecutive patients undergoing CABG at the Department of Cardiothoracic Surgery, University Hospital Magdeburg, Magdeburg, Germany. To be included in the study, patients had to be scheduled for elective CABG requiring cardiopulmonary bypass. Patients were excluded if they had any of the following: a history of AF; atrial flutter or atrial tachycardias; required the use of antiarrhythmic drug classes I and III; significant aortic, mitral and tricuspid valve diseases; or severe heart failure (left ventricular ejection fraction <30%). Of the patients, 46 were smokers (active cigarette smokers and/or a history of CS), and the remaining 49 were non‐smokers (NS). Former smokers were abstinent for 19 (13) years. The amount of nicotine misuse was characterised by “pack year”, defined as one pack of cigarettes (20 cigarettes) per day throughout a year. Arterial hypertension was defined as a history of relative resistance (RR) values >140/90 mm Hg at rest. Hyperlipoproteinaemia was defined as low‐density lipoprotein‐cholesterol level ⩾2.6 mmol/l. All patients gave written consent to participate in the study and table 1 shows their baseline characteristics. Table 2 shows the clinical characteristics of current and former smokers. A total of 130 patients were initially screened for the study. In all 5 patients refused to participate and 30 patients were excluded due to significant concomitant valve diseases. The remaining 95 (73%) patients were included in this study. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Table 1 Clinical characteristics of patients.
| Characteristic | Non‐smoker n = 49 | Smoker n = 46 | p Value |
|---|---|---|---|
| Age (years) | 70 (5) | 67 (5) | 0.031 |
| Diabetes mellitus, n (%) | 22 (44) | 18 (39) | 0.569 |
| Hyperlipoproteinaemia, n (%) | 46 (94) | 41 (89) | 0.405 |
| Hypertension, n (%) | 46 (94) | 40 (87) | 0.250 |
| Three‐vessel coronary artery disease, n (%) | 40 (82) | 39 (85) | 0.202 |
| Myocardial infarction, n (%) | 23 (47) | 17 (37) | 0.325 |
| NYHA functional class | 2.1 (1.1) | 2.2 (1.1) | 0.239 |
| Ejection fraction (%) | 57 (15) | 56 (15) | 0.633 |
| Left atrial diameter (cm) | 4.2 (0.3) | 3.8 (0.5) | 0.752 |
| P‐wave width (ms) | 70 (20) | 80 (30) | 0.086 |
| Creatinine before surgery (μmol/l) | 81.3 (20.8) | 86.2 (19.9) | 0.246 |
| C reactive peptide before surgery (mg/l) | 13.6 (23.7) | 11.5 (18.6) | 0.635 |
| C reactive peptide after surgery (mg/l) | 56.7 (36.1) | 73.1 (39.9) | 0.034 |
| Treatment before heart surgery: | |||
| β blocker, n (%) | 27 (55) | 17 (37) | 0.076 |
| Digitalis, n (%) | 9 (18) | 7 (15) | 0.682 |
| Calcium‐channel blocker, n (%) | 9 (18) | 5 (10) | 0.303 |
| ACE inhibitor/AT1‐antagonists, n (%) | 30 (61) | 27 (59) | 0.801 |
| Type of heart surgery: | |||
| CABG, n (%) | 49 (100) | 46 (100) | |
| Postoperative AF (%) | 12 (25) | 11 (24) | 0.948 |
| Duration of AF (min) | 118 (279) | 96 (252) | 0.296 |
| Amount of atrial fibrosis (vol %) | 24.5 (7.3) | 23.3 (6.5) | 0.407 |
AF, atrial fibrillation; AT1, angiotensin II type I receptor blocker; CABG, coronary artery bypass grafting; NYHA, New York Heart Association.
Table 2 Clinical characteristics of former and active smokers.
| Characteristic | Former smoker n = 30 | Active smoker n = 16 | p Value |
|---|---|---|---|
| Age (years) | 67 (5) | 67 (4) | 1.0 |
| Diabetes mellitus, n (%) | 12 (40) | 6 (37) | 0.839 |
| Hyperlipoproteinaemia, n (%) | 27 (90) | 14 (88) | 0.321 |
| Hypertension, n (%) | 28 (93) | 12 (75) | 0.067 |
| Three–vessel coronary artery disease, n (%) | 26 (86) | 13 (81) | 0.856 |
| Myocardial infarction, n (%) | 11 (37) | 6 (38) | 0.615 |
| NYHA functional class | 1.9 (1.2) | 2 (1.1) | 1.0 |
| Ejection fraction (%) | 55 (17) | 57 (10) | 1.0 |
| Left atrial diameter (cm) | 3.9 (0.7) | 4.1 (0.44) | 1.0 |
| P‐wave width (ms) | 80 (25) | 80 (30) | 1.0 |
| Creatinine before surgery (μmol/l) | 89.6 (20.6) | 80.6 (17.9) | 0.503 |
| C reactive peptide before surgery (mg/l) | 10.8 (17.9) | 12.6 (20.5) | 1.0 |
| C reactive peptide after surgery (mg/l) | 76.6 (35.5) | 66.9 (39.6) | 1.0 |
| Treatment before heart surgery: | |||
| β Blocker, n (%) | 10 (33) | 7 (44) | 0.166 |
| Digitalis, [n (%) | 5 (17) | 2 (13) | 0.862 |
| Calcium‐channel blocker, n (%) | 2 (7) | 3 (19) | 0.321 |
| ACE inhibitor/AT1‐antagonists, n (%) | 15 (50) | 12 (75) | 0.249 |
| Type of heart surgery: | |||
| CABG, n (%) | 30 (100) | 16 (100) | |
| Postoperative AF (%) | 7 (23) | 4 (25) | 0.976 |
| Duration of AF (min) | 36.1 (29.3) | 11.3 (9.4) | 1.0 |
| Amount of atrial fibrosis (vol %) | 21.7 (5.8) | 26.3 (6.9) | 0.103 |
AF, atrial fibrillation; AT1, angiotensin II type I receptor; CABG, coronary‐artery bypass grafting; NYHA, New York Heart Association.
Study protocol
All patients were hospitalised 1 day before surgery. After a clinical examination, a 12‐lead ECG was recorded for each patient and routine blood samples were taken to determine white and red blood cell counts, hepatic and renal function and C‐reactive protein (CRP) level. All surgical procedures were performed using extracorporal circulation. During the surgery, myocardial protection was provided by cold cardioplegia (Bretschneider solution) to every patient. After the procedure, all patients were transferred to an intensive care unit where they were monitored continuously for at least 72 h. A 12‐lead ECG was recorded daily and in case of suspected arrhythmias during the period of hospitalisation. The P‐wave duration was assessed preoperatively using measurements from two independent observers in lead II from a standard 12‐lead surface ECG (50 mm/s paper speed). AF episodes lasting <5 min were counted as an episode of postoperative AF, and treatment depended on the clinical presentation of the patient. Preoperative medication with β‐blockers was continued after surgery. The remaining patients received no specific postoperative medical prophylaxis for AF. All patients were discharged from hospital approximately 11 days after surgery.
Quantification of interstitial fibrosis
Fibrous tissue was quantified as described previously.12 Tissue samples (one or two entire cross sections, depending on the amount of tissue available) of the right atrial appendages from all 95 patients were fixed in formalin and embedded in paraffin wax. Deparaffinised sections were stained with H&E and van Gieson's elastic stain (EvG). The interstitial fibrosis was quantitatively analysed with point counting on EvG‐stained paraffin wax sections; fibrotic areas rich in elastin and collagen stained black and bright red, respectively. A 144‐point multipurpose microgrid was fitted in a 12.5× ocular of a microscope equipped with the 16× objective, yielding a test field size of 0.289 mm2 at specimen level. Points falling on fibrotic areas were counted in 15 randomly selected fields per specimen. The volume percentage of interstitial fibrosis was estimated using mean number of points per field, in that 144 points per field were equal to 100%. The pathologist who read the biopsy specimens and quantitated the amount of fibrosis was unaware of the personal and clinical data of the patients.
Immunohistochemistry
The expression of collagens I, III and IV was studied immunohistochemically in atrial appendages obtained from 10 smokers (8 men and 2 women; mean (SD) age 61.2 (10) years) and 10 NS (7 men and 3 women; mean (SD) age 59.7 (9.6) years). Immunostaining was performed with antibodies directed against collagen type I (dilution 1:100, monoclonal; Biermann, Hamburg, Germany), collagen type III (1:20, polyclonal; Quartett, Berlin, Germany) and collagen type IV (1:500, monoclonal; Sigma, Deisenhofen, Germany). Immunostaining with anti‐collagen type III antibodies necessitated antigen retrieval with protease 1 (20 min, 37°C; Ventana, Strasbourg, France), and with anti‐collagen type IV antibodies with papain (8 min, room temperature). Immunoreactions were visualised with the avidin–biotin complex method applying a Vectastain ABC‐alkaline phosphatase kit (Alexis Deutschland, Grünberg, Germany). FastRed (Zytomed, Berlin, Germany) served as chromogen. Specimens were counterstained with haematoxylin. The specificity of immunostaining was controlled by omitting the primary antibody. Immunostaining was categorised into absent (0), mild (+), moderate (++) and strong (+++).
Organotypic atrial tissue culture
Atrial tissue samples (right atrial appendage) from NS (n = 8) were obtained during open heart surgery and directly processed for culturing. The tissue was immediately sliced (350 μm), and between five and nine slices were placed on top of 0.02 μm Anopore membrane tissue culture inserts (25 mm, Nunc, Wiesbaden, Germany). Four of these inserts were placed in a Petri dish (8 cm diameter; Nunc, Wiesbaden, Germany), which was filled with culture medium. (10 mmol/l Hepes buffer, pH 7.4, 50% low essential medium (Sigma), 20% Hanks' balanced salt (Gibco), 20% horse serum (Gibco), 6 mmol/l glucose, 1× non‐essential amino acids (Gibco), 1% OPI media supplement (Sigma) and 82.5 μmol/l refobacin (Merck, Darmstadt, Germany)). Tissue slices were cultured for 24 h at 37°C. To determine the effect of nicotine, atrial tissue of each individual patient was divided into three pieces and cultured in the presence of 0, 185 and 740 nmol/l nicotine base, respectively.
Quantitative PCR
Total RNA was prepared as described recently14 by applying the method of Chomczynski and Sacchi.15 Quantitative PCR was performed using the iCycler (BioRad, Munich, Germany). All samples were analysed in triplicate. A 25 μl reaction mixture consisted of 1× HotStartTaq Master Mix (Qiagen, Hilden, Germamy), 0.5 μl of a 1:1000 dilution of SYBR‐Green I (Molecular Probes, Eugene,OR, USA), 1 μl complementary DNA and 0.5 μmol/l of specific primers for collagen I (upstream: 5′‐AATCACCTGCGTACAGAACG; downstream: 5′‐CATAGCCATAAGACAGCTGG), collagen III (upstream: 5′‐CATCTTGGTCAGTCCTATGC; downstream: 5′‐TGGTTGTCCTGGAATACCAG) and gylceraldehyde‐3‐phosphate dehydrogenase (GAPDH; upstream: 5′‐TCCAAAATCAAGTGGGGCGATGCT; downstream: 5′‐ACCACCTGGTGCTCAGTGTGACCC).
Initial denaturation at 95°C for 15 min was followed by 40 cycles with denaturation at 95°C for 30 s annealing at 58°C for 30 s, and elongation at 72°C for 30 s. Quantities of gylceraldehyde‐3‐phosphate dehydrogenase mRNA were used to normalise complementary DNA contents. The fluorescence intensity of the double‐strand‐specific SYBR‐Green I, which reflected the amount of PCR product actually formed, was read real‐time at the end of each elongation step. Amounts of specific initial template mRNA were then calculated by determining the time point at which the linear increase of sample PCR product started, relative to the corresponding points of a standard curve; these are given as arbitrary units.
Statistics
All values are expressed as mean (SD). Continuous variables were compared by means of the unpaired Student's t test and Wilcoxon's test. The χ2 test was used to assess the association between postoperative AF and qualitative variables (gender, smoking, diabetes, hypertension, hyperlipoproteinaemia, myocardial infarction, medicaltreatment). Univariate and multivariate regression analyses were used to assess the association between the amount of fibrosis and (quantitative) clinical parameters (age, left ventricular ejection fraction, CRP, P‐wave duration, pack years). The Pearson coefficient was used to determine the correlation between quantitative parameters (age, amount of fibrosis, left ventricular ejection fraction, NYHA class, creatinine level, CRP, P wave duration, atrial diameter). A value of p<0.05 was considered to be significant.
Results
Patients' characteristics
Table 1 summarises the clinical characteristics of the patients. With exception of age, clinical parameters (left ventricular ejection fraction, severity of coronary artery disease, perioperative parameters, medical treatment and so on) were comparable in smokers and NS. Postoperative CRP levels were higher in smokers (table 1). Clinical parameters were comparable in current and former smokers (table 2).
Atrial fibrosis
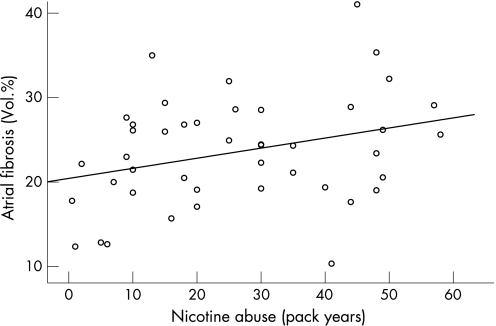
The volume percentage of atrial fibrosis, as quantified by point counting on EvG‐stained tissue sections,12 ranged from 10.3% to 48.3% (24% (6.5%)). The absolute amount of right atrial fibrosis was similar in smokers and NS (24.5% (7.3%) vs. 23.3% (6.5%); p = ns) although smokers were significantly younger (table 1). Furthermore, in smokers, pack years of nicotine misuse (31(14) pack years; range 7–58 pack years) was the only parameter, which was related to the amount of right atrial fibrosis (r = 0.311; p<0.05; fig 1) using multivariate regression analysis (table 3). All remaining quantitative (metric) parameters (age, left ventricular ejection fraction, CRP, P‐wave duration) were not related to the amount of atrial fibrosis using regression analysis (tables 3 and 4). Amounts of fibrous tissue were not different in active smokers compared with former smokers (table 2). Former smokers had a mean nicotine misuse of 23(15) pack years. The amount of fibrosis was not inversely correlated with the time duration after cessation of smoking (19 (13) years).
Figure 1 Correlation between nicotine misuse (pack years) and amount of atrial fibrosis (r = 0.31; p<0.05).
Table 3 Multivariate regression analysis‐dependent variable: amount of atrial fibrosis.
| Characteristics | Coefficient | p Value |
|---|---|---|
| Smoker (n = 36) | ||
| Constant | 15.647 | 0.15 |
| Age (years) | 0.27 | 0.93 |
| Ejection fraction (%) | –0.33 | 0.63 |
| C reactive peptide before surgery (mg/l) | 0.37 | 0.54 |
| P‐wave duration (ms) | 47.54 | 0.29 |
| Pack years | 0.16 | 0.30 |
| After reduction | ||
| Constant | 20.07 | <0.001 |
| Pack years | 0.156 | 0.026 |
| Non‐smoker (n = 45) | ||
| Constant | 11.266 | 0.49 |
| Age (years) | 0.22 | 0.33 |
| Ejection fraction (%) | 0.03 | 0.71 |
| C reactive peptide before surgery (mg/l) | –0.09 | 0.04 |
| P‐wave duration (ms) | –39.84 | 0.38 |
| After reduction | ||
| Constant | 25.33 | <0.001 |
| C reactive peptide before surgery (mg/l) | –0.08 | 0.05 |
Table 4 Correlation of clinical parameters with amount of atrial fibrosis.
| Characteristics | Non‐smoker | Smoker | ||||
|---|---|---|---|---|---|---|
| Correlation | p Value | n | Correlation | p Value | n | |
| Age (years) | 0.153 | 0.293 | 49 | 0.031 | 0.839 | 45 |
| Ejection fraction (%) | –0.074 | 0.611 | 49 | 0.082 | 0.592 | 45 |
| P‐wave width (ms) | –0.036 | 0.810 | 48 | 0.223 | 0.161 | 41 |
| Creatinine before surgery | –0.067 | 0.646 | 49 | 0.139 | 0.380 | 42 |
| C reactive peptide before surgery (mg/l) | –0.298 | 0.045 | 46 | 0.074 | 0.645 | 41 |
| C reactive peptide after surgery (mg/l) | 0.109 | 0.462 | 45 | –0.116 | 0.459 | 43 |
| NYHA functional class | –0.038 | 0.795 | 49 | 0.246 | 0.103 | 45 |
| Left atrial diameter (cm) | –0.394 | 0.183 | 13 | 0.190 | 0.380 | 10 |
NYHA, New York Heart Association.
In the overall study population, the amount of atrial fibrosis correlated with patient age (r = 0.18; p<0.05). Furthermore, there was a trend between atrial fibrosis and time after previous myocardial infarction (r = 0.43; p = 0.06) and there was an inverse relationship between preoperative CRP level and amount of fibrosis (r = –0.29; p<0.05). All remaining quantitative (metric) parameters were not related to the amount of atrial fibrosis in NS (tables 3 and 4).
Nicotine and collagen expression
Next we investigated the expression pattern of collagen in atrial appendages. Using immunohistochemistry we found collagens I, III and IV in atrial tissue obtained from smokers and NS (fig 2). Collagen type I was present in the cytoplasm of cardiomyocytes and smooth muscle cells of vessel walls of all patients, as well as in the interstitium of 3 (30%) NS and 3 (30%) smokers. The overall intensity of cytoplasmic immunostaining for collagen type I was decreased in smokers (fig 2). The overall expression pattern of collagens III and IV did not differ between smokers and NS. However, atrial fibrotic tissue immunostained for collagen III in every patient (100%) and the extent of collagen III immunoreactivity correlated with the amount of atrial fibrosis. Collagen IV was found at the basal lamina of cardiomyocytes of smokers and NS, and in the fibrotic areas of only two smokers.
Figure 2 Atrial tissue of smokers (left lane) and non‐smokers (NS; right lane) immunostained with antibodies directed against collagen I, III and IV. Collagen type I was present in the cytoplasm of cardiomyocytes. The overall intensity of cytoplasmic immunostaining for collagen type I was decreased in smokers. The overall expression pattern of collagens III and IV did not differ between smokers and NS. However, atrial fibrotic tissue largely consisted of collagen III, and the extent of collagen III immunoreactivity correlated with the amount of atrial fibrosis in smokers. Collagen IV was found at the basal lamina of cardiomyocytes of smokers and NS. Haematoxylin counterstain; original magnifications ×200.
Finally we investigated the direct effect of nicotine base on atrial collagen expression in an organotypic tissue culture model. Human atrial tissue slices (n = 8) were cultured in the presence of 185 or 740 nmol/l nicotine base. In that model, atrial tissue slices can be kept viable up to 7 days.16,17 In vitro, the nicotine base induced a substantial increase in atrial collagen III expression. After 24 h incubation, levels of collagen III mRNA increased more than sevenfold with 30 ng/ml nicotine base (740% (261%) compared with control; p<0.05) and 10‐fold with 120 ng/ml nicotine base (1274% (744%) compared with control; p<0.05; fig 3A). The collagen I mRNA expression was significantly increased after incubation with 120 ng/ml nicotine base only (197% (42%) compared with control; p<0.05; fig 3B). Collagen IV mRNA was not studied since immunohistology did not show substantial differences in the amounts of collagen IV.
Figure 3 (A): Expression of collagen III mRNA in human atrial tissue after 24 h of incubation with different concentrations of nicotine base and untreated controls. (B) Effect of nicotine base on atrial expression of collagen I mRNA after 24 h. *p<0.05 versus untreated controls.
Atrial fibrosis and postoperative AF
About 24% of all patients developed AF within 72 h after cardiac surgery (table 5). The overall incidence of AF was similar in smokers and NS (table 6). Clinical parameters were comparable in patients with and without AF (table 5). As shown in a previous study, only atrial fibrosis was related to the occurrence of postoperative AF in all patients (p<0.05). The amount of interstitial fibrosis (% of tissue volume) was increased in patients with postoperative AF (AF: 22.9% (6.2%) versus 27.0% (8.2%); p<0.05). No other variable was related to the occurrence of postoperative AF (table 7). Postoperative CRP levels were comparable in patients with and without postoperative AF (AF: 63 (37) mg/l vs sinus rhythm: 68 (39) mg/l; p = ns). There were no significant wound infections or postoperative myocardial infarctions or major adverse events in any patient. The duration of AF episodes did not correlate with the amount of fibrosis.
Table 5 Characteristics of the patients with and without postoperative atrial fibrillation.
| Characteristic | No postoperative AF (n = 72) | Postoperative AF (n = 23) | p Value |
|---|---|---|---|
| Age (years) | 69 (5) | 69 (5) | 0.922 |
| Diabetes mellitus, n (%) | 29 (40) | 11 (48) | 0.523 |
| Hyperlipoproteinaemia, n (%) | 66 (92) | 21 (91) | 0.957 |
| Hypertension, n (%) | 65 (90) | 21 (91) | 0.884 |
| Three‐vessel coronary artery disease n (%) | |||
| Myocardial infarction n (%) | 28 (39) | 12 (52) | 0.261 |
| NYHA functional class | 2 (1.1) | 2.4 (1) | 0.175 |
| Ejection fraction n (%) | 56 (14) | 58 (15) | 0.650 |
| Left atrial diameter (cm) | 4.0 (0.5) | 4.0 (0.4) | 0.940 |
| P‐wave width (ms) | 77 (26) | 71 (25) | 0.324 |
| Pack years | 24 (16.8) | 31 (17.1) | 0.234 |
| Creatinine before surgery (μmol/l) | 82 (20) | 89 (21) | 0.116 |
| C reactive peptide before surgery (mg/l) | 10 (16) | 19 (32) | 0.221 |
| C reactive peptide after surgery (mg/l) | 63 (37) | 68 (39) | 0.611 |
| Treatment before heart surgery: | |||
| β blocker, n (%) | 34 (47) | 10 (44) | 0.754 |
| Digitalis, n (%) | 11 (15) | 5 (22) | 0.471 |
| Calcium‐channel blocker, n (%) | 9 (13) | 5 (22) | 0.276 |
| ACE‐inhibitor/AT1‐antagonists n (%) | 41 (57) | 16 (70) | 0.282 |
| Duration of AF (min) | – | 74 (203) | |
| Amount of atrial fibrosis (vol %) | 23 (6) | 27 (8) | 0.020 |
AF, atrial fibrillation; AT1, angiotensin II type I receptor blocker; CABG, coronary artery bypass grafting; NYHA, New York Heart Association.
Table 6 Characteristics of the patients with postoperative atrial fibrillation.
| Characteristic | Non‐smoker (n = 12) | Smoker (n = 11) | p Value |
|---|---|---|---|
| Age (years) | 70 (4.7) | 68 (4.8) | 0.346 |
| Diabetes mellitus, n (%) | 7 (58) | 4 (36) | 0.292 |
| Hyperlipoproteinaemia, n (%) | 11 (92) | 10 (91) | 0.949 |
| Hypertension, n (%) | 11 (92) | 10 (91) | 0.949 |
| Three‐vessel coronary artery disease n (%) | |||
| Myocardial infarction, n (%) | 7 (58) | 5 (45) | 0.537 |
| NYHA functional class | 2.3 (1.1) | 2.5 (1) | 0.629 |
| Ejection fraction (%) | 59.5 (13) | 56.3 (17) | 0.607 |
| P‐wave width (ms) | 63 (20) | 80 (27) | 0.082 |
| Creatinine before surgery (μmol/l) | 85 (20) | 93 (23) | 0.355 |
| C reactive peptide before surgery (mg/l) | 27 (41) | 9 (5) | 0.153 |
| C reactive peptide after surgery (mg/l) | 58 (26) | 78 (48) | 0.221 |
| Treatment before heart surgery: | |||
| β blocker, n (%) | 6 (50) | 4 (36) | 0.510 |
| Digitalis, n (%) | 2 (17) | 3 (27) | 0.538 |
| Calcium‐channel blocker, n (%) | 3 (25) | 2 (18) | 0.692 |
| ACE‐inhibitor/AT1‐antagonists, n (%) | 7 (58) | 9 (81) | 0.221 |
| Amount of atrial fibrosis (vol %) | 26.4 (10.1) | 27 (6) | 0.785 |
AT1, angiotensin II type I receptor blocker; CABG, coronary artery bypass grafting; NYHA, New York Heart Association.
Table 7 Multivariate regression analysis‐dependent variable: postoperative atrial fibrillation.
| Characteristics | Coefficient | p Value |
|---|---|---|
| Smoker (n = 39) | ||
| Constant | –11.130 | <0.001 |
| Age (years) | 0.104 | 0.29 |
| Ejection fraction (%) | –0.17 | 0.83 |
| C reactive peptide after surgery (mg/l) | 0.15 | 0.17 |
| P‐wave duration (ms) | –14.23 | 0.45 |
| Amount of atrial fibrosis (vol %) | 0.135 | 0.08 |
| Pack years | 0.03 | 0.38 |
| After reduction | ||
| Constant | –3.92 | 0.02 |
| Amount of atrial fibrosis (vol %) | 0.121 | 0.05 |
| Non‐smoker (n = 47) | ||
| Constant | –3.058 | 0.60 |
| Age (years) | 0.02 | 0.80 |
| Ejection fraction (%) | 0.03 | 0.71 |
| C reactive peptide after surgery (mg/l) | 0.002 | 0.84 |
| Amount of atrial fibrosis (vol %) | 0.052 | 0.28 |
| P‐wave duration (ms) | –27.135 | 0.12 |
| After reduction | ||
| Constant | –1.07 | 0.001 |
Discussion
To the best of our knowledge, this is the first prospective study to determine the relationship between the amount of atrial fibrosis and CS. We show here that nicotine misuse (pack years) accelerates atrial collagen accumulation, leading to symptomatic atrial fibrosis. This finding was further supported by results obtained in an organotypic atrial tissue culture. Nicotine base induced predominantly collagen III mRNA expression in a concentration‐dependent manner, which is the major constituent of atrial fibrosis in smokers.
Myocardial effects of nicotine
In this study we show the impact of CS on atrial collagen accumulation. This effect of CS seems to be related to the extent of nicotine misuse (pack years). At the molecular level, the effect of CS on atrial myocardium depends on the concentration of nicotine base. Nicotine concentrations between 60 and 600 nmol/l have been found in smokers.8 Thus, the concentration of 185 nmol/l nicotine base used in this study, with its substantial effects on collagen III expression (in contrast with the insignificant effects on collagen I), may explain the immunohistological collagen pattern found in CS, in whom collagen III is increased, whereas collagen I seems to be decreased. High concentrations of nicotine base (>600 nmol/l) induce collagen I expression as well. However, such concentrations of nicotine base are unrealistic chronically in CS. Nevertheless, the in vitro analysis of collagen mRNA levels may not necessarily reflect the slow mechanism of collagen fibre deposition in vivo. However, our findings in atrial tissue are supported by previous results showing that nicotine induces pulmonary collagen I and III mRNA expression in the fetal lung via α7 receptors, which are strongly expressed on fibroblasts.18,19 Activation of the α7 receptor by exposure to maternal nicotine is believed to cause pulmonary fibrosis of the fetal lung.18,19 In the cardiovascular system, chronic exposure to nicotine has been found to increase aortic stiffness,3 to induce moderate interstitial fibrosis in the ventricles and, thereby, to increase chamber stiffness.20,21 In addition, CS is associated with increased inflammatory markers such as high‐sensitivity CRP levels, which have also been reported to be increased in patients with AF.2,22 Nevertheless, CRP levels did not discriminate CS from NS in this study, and there was a negative correlation between CRP and amount of fibrosis in NS. These findings may have been influenced by the high proportion of severe artherosclerosis in the present patient cohort and the rather high incidence of concomitant diseases such as hypertension and hyperlipoproteinaemia. Due to the limited number of patients in this study, the inverse correlation between preoperative CRP levels and atrial fibrosis in NS has to be interpreted with caution. The absence of a positive correlation between the two variables clearly supports the fact that the development of atrial fibrosis is not due to secondary CRP‐dependent proinflammatory mechanisms, but rather related to other profibrotic substances such as nicotine.
CS is well known to induce cardiac ischaemia because of artherosclerosis of coronary arteries or vascular spasms, which may lead to localised necrosis and replacement fibrosis. In contrast with myocardium, atrial oxygen supply largely depends on oxygen diffusion from atrial chambers, and, therefore, atrial ischaemia induced by direct vascular effects of CS seems unlikely to explain the observed diffuse pattern of fibrosis in atrial tissue specimen obtained from smokers. Furthermore, the extent of coronary artery disease was not different in CS compared with NS, suggesting a limited impact of coronary vascular disease on the development of atrial fibrosis in this study.
Besides the profibrotic effects, nicotine inhibits ionic currents in a concentration‐dependent manner.10,23 Nicotine at 300 μmol/l inhibits ICa at 10 mV by 29.3% (2.4%) and blocks Ica at 1 mmol/l.23 Nicotine at 100 µm also inhibits the delayed rectifier K+ current at 60 mV by 42.7% (3.0%), and at 30 μmol/l inhibits the inwardly rectifying K+ current at –110 mV by 43.0(2.5%).23 Recent animal experiments have also shown that the nicotine base has significant effects on atrial refractoriness and facilitates the inducibility of AF especially in older animals. Therefore, the impact of CS on postoperative AF may be due to induced structural atrial changes (fibrosis) and by direct electrophysiological effects. However, in this study, the presence of structural changes (fibrosis), and not CS by itself, was the predictor for postoperative AF.
Atrial fibrosis and postoperative AF
Atrial fibrosis isolates groups of atrial myocytes and individual myocytes, and thereby, interstitial fibrosis may cause inhomogeneities in intra‐ and interatrial conduction.12,24,25 Initially, subsequent changes in atrial conduction may be subtle, and therefore not detectable on the surface ECG.13,26 Previous studies have clearly shown that atrial fibrosis may provide a structural substrate for AF: areas of fibrotic tissue are in macro re‐entry circuits during AF, increased amounts of atrial fibrosis were found in patients with AF11 and the amount of atrial fibrosis increases with patient age.12 Similar to our findings, previous studies have also revealed an association between age, atrial fibrosis, surface P‐wave duration and postoperative AF.12,13,26,27,28 In addition to replacement fibrosis, the presence of reactive cardiac fibrosis, possibly induced by increased levels of angiotensin II, endothelin, transforming growth factor β and so on has been postulated.14,24,29 Previous studies have also shown that mechanical stretch as well as the presence of AF influences the ratio of collagen III to collagen I.30,31 Boldt et al31 could show that mechanical stress induced by mitral valve diseases increases the amount of collagen III in left atrial tissue samples. Patients with AF and mitral valve diseases had increased collagen I and collagen III levels. Left atrial collagen I expression was predominant in patients with only AF. In contrast with these previous results, and to limit the described effects of concomitant cardiovascular diseases on myocardial collagen expression, patients with valve diseases, advanced heart failure and/or AF were excluded in our analysis. Thus, this study adds new pathophysiological information to explain the well‐described proarrhythmic potential of CS and chronic nicotine consumption with regard to atrial arrhythmia.5,6,7,8,9,10 An interesting finding of this study is that there was an inverse relationship between CRP level and amount of atrial fibrosis in NS. This may suggest that inflammatory mechanisms are not primarily involved in the development of atrial fibrosis in NS with coronary artery disease and sinus rhythm.
Nevertheless, the pathogenesis of postoperative AF is multifactorial and not directly comparable to spontaneous episodes of AF in patients not undergoing surgery. Several factors that have previously been characterised such as premature beats, increased catecholamine levels, electrolyte disturbances and so on contribute to the occurrence of postoperative arrhythmias.27,28 Therefore, the relationship between separate factors/parameters and the occurrence of postoperative AF remains weak.
Limitations
A limitation of this study is that direct electrophysiological effects of CS were not assessed and that systemic nicotine concentrations were not determined. However, the organotypic culture model facilitates the direct analysis of the molecular effects of nicotine base in adult atrial tissue. It is of note that functional experiments are impossible in atrial tissue in vivo, because repetitive atrial biopsies are unfeasible. Thus, our tissue model allows, for the first time, molecular experiments using human atrial tissue samples. Nevertheless, in vitro analyses of mRNA levels may not necessarily reflect the slow process of interstitial collagen depositions in vivo. No comment can be made about different effects of CS on right and left atrial tissue, because left atrial appendages were not available during the study. However, the principle responses of atrial tissue are similar in the right and left atria at the molecular level, although quantitative differences in cellular signalling seem to exist. With regard to the nicotine base, the molecular response in right atrial tissue was dramatic in this study (up to 10‐fold increase in collagen III mRNA), which clearly supports the impact of CS on collagen accumulation. Besides nicotine, cigarette smoke consists of several hundred substances and metabolites. Therefore, it is likely that substances other than nicotine contribute to myocardial alterations in CS in vivo. Nevertheless, the observed differences in atrial biopsies from smokers and NS are not in the range of high significance (p<0.01). Thus, some of our results have to be interpreted with caution since the power of this study is limited. Further studies are necessary to confirm our results in a larger cohort of patients.
EvG and point counting was used to quantify the amount of atrial fibrosis,12 and immunohistochemistry to study collagen types contributing to the structural change. However, since immunohistochemistry is of limited use for the quantification of collagen amounts, western blotting is preferred by recent studies.31
Conclusion
Besides the well‐known effects of CS on endothelial function and atherosclerosis, this is the first prospective study showing the impact of CS on human atrial tissue. In CS, nicotine contributes to the development of atrial fibrosis in patients with coronary artery disease. Previous studies have shown that atrial fibrosis can provide an arrhythmogenic substrate, which may increase the likelihood of the occurrence of atrial arrhythmias, including postoperative AF. This nicotine effect merits further investigations in larger clinical and epidemiological studies.
Acknowledgements
This work was supported by grants from the “Kultusministerium des Landes Sachsen‐Anhalt, Germany” (3517 A/0603M) and by the “Bundesministerium für Bildung und Forschung, Germany” (Kompetenznetz Vorhofflimmern, grants 01GI0204 and 01ZZ0407).
Abbreviations
AF - atrial fibrillation
CABG - coronary artery bypass grafting
CRP - C reactive protein
CS - cigarette smoking
EvG stain - van Gieson's elastic stain
mRNA - messenger RNA
NS - non‐smokers
NYHA - New York Heart Association
Footnotes
Competing interests: None.
References
- 1.Benowitz N L, Gourlay S G. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 1997291422–1431. [DOI] [PubMed] [Google Scholar]
- 2.Fröhlich M, Sund M, Löwel H.et al Independent association of various smoking characteristics with markers of systemic inflammation in men: results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur Heart J 2003241365–1372. [DOI] [PubMed] [Google Scholar]
- 3.Lartaud‐Idjouadiene I, Lompre A M, Kieffer P.et al Cardiac consequences of prolonged exposure to an isolated increase in aortic stiffness. Hypertension 19993463–69. [DOI] [PubMed] [Google Scholar]
- 4.Qiao Q, Tervahauta M, Nissinen A.et al Mortality from all causes and from coronary heart disease related to smoking and changes in smoking during a 35‐year follow‐up of middle‐aged Finnish men. Eur Heart J 2000211621–1626. [DOI] [PubMed] [Google Scholar]
- 5.Van Berkel T F M, Boersma H, De Baquer D.et al Registration and management of smoking behaviour in patients with coronary heart disease. The EUROASPIRE Survey. Eur Heart J 1999201630–1637. [DOI] [PubMed] [Google Scholar]
- 6.Mehta M C, Jain A C, Mehta A.et al Cardiac arrhythmias following intravenous nicotine: experimental study in dogs. J Cardiovasc Pharmacol Ther 19972291–298. [DOI] [PubMed] [Google Scholar]
- 7.Yashima M, Ohara T, Cao J M.et al Nicotine increases ventricular vulnerability to fibrillation in hearts with healed myocardial infarction. Am J Physiol Heart Circ Physiol 2000278H2124–H2133. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi H, Omichi C, Miyauchi Y.et al Age‐related sensitivity to nicotine for inducible atrial tachycardia and atrial fibrillation. Am J Physiol Heart Circ Physiol 2003285H2091–H2098. [DOI] [PubMed] [Google Scholar]
- 9.Stewart P M, Catterall J R. Chronic nicotine ingestion and atrial fibrillation. Br Heart J 198554222–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Shi H, Zhang L.et al Nicotine is a potent blocker of the cardiac A‐type K+ channels. Circulation 20001021165–1171. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Fareh S, Leung T K.et al Promotion of atrial fibrillation by heart failure in dogs: electrical remodeling of a different sort. Circulation 199910087–95. [DOI] [PubMed] [Google Scholar]
- 12.Goette A, Juenemann G, Peters B.et al Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res 200254390–396. [DOI] [PubMed] [Google Scholar]
- 13.Zaman A G, Arehbold R A, Helft G.et al Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation 20001011403–1408. [DOI] [PubMed] [Google Scholar]
- 14.Goette A, Staack T, Röcken C.et al Increased expression of extracellular signal‐regulated kinase and angiotensin‐converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol 2000351669–1677. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Anal Biochem 1987162156–159. [DOI] [PubMed] [Google Scholar]
- 16.Bukowska A, Lendeckel U, Hirte D.et al Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. J Cell Mol Life Sci 200663333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goette A, Bukowska A, Müller C.et al Tachycardia‐induced cellular hypertrophy: an in‐vitro pacing model to study molecular biology of cardiomyocytes. Heart Rhythm 20041(Suppl 1)S155 [Google Scholar]
- 18.Sekhon H S, Jia Y, Raab R.et al Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 1999103637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekhon H S, Keller J A, Proskocil B J.et al Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 20022631–41. [DOI] [PubMed] [Google Scholar]
- 20.Rajiyah G, Agarwal R, Avendano G.et al Influence of nicotine on myocardial stiffness and fibrosis during chronic ethanol use. Alcohol Clin Exp Res 199620985–989. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki J, Bayna E, Dalle Molle E.et al Nicotine inhibits cardiac apoptosis induced by lipopolysaccharide in rats. J Am Coll Cardiol 200341482–488. [DOI] [PubMed] [Google Scholar]
- 22.Chung M K, Martin D O, Sprecher D.et al C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 20011042886–2891. [DOI] [PubMed] [Google Scholar]
- 23.Satoh H. Modulation by nicotine of the ionic currents in guinea pig ventricular cardiomyocytes. Relatively higher sensitivity to Ikr and IKI. Vasc Pharmacol 20023955–61. [DOI] [PubMed] [Google Scholar]
- 24.Goette A, Lendeckel U. Non‐channel drug targets in atrial fibrillation. Pharmacol Ther 200410217–36. [DOI] [PubMed] [Google Scholar]
- 25.Nitta T, Imura H, Bessho R.et al Wavelength and conduction inhomogeneity in each atrium in patients with isolated mitral valve disease and atrial fibrillation. J Cardiovasc Electrophysiol 199910521–528. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg J S, Zelenkofske S, Wong S C.et al Value of the P‐wave signal‐averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993882618–2622. [DOI] [PubMed] [Google Scholar]
- 27.Aranki S F, Shaw D P, Adams D H.et al Predictors of atrial fibrillation after coronary artery surgery: current trends and impact on hospital resources. Circulation 199694390–397. [DOI] [PubMed] [Google Scholar]
- 28.Buxton A E, Josephson M E. The role of P‐wave duration as a predictor of postoperative atrial arrhythmias. Chest 19818068–73. [DOI] [PubMed] [Google Scholar]
- 29.Campbell S E, Katwa L C. Angiotensin II stimulated expression of transforming growth factor‐beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 1997291947–1958. [DOI] [PubMed] [Google Scholar]
- 30.Carver W, Nagpal M L, Nachtigal M.et al Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res 199169116–122. [DOI] [PubMed] [Google Scholar]
- 31.Boldt A, Wetzel U, Lauschke J.et al Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 200490400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]