Abstract
Background
Methadone is prescribed to heroin addicts to decrease illicit opioid use. Prolongation of the QT interval in the ECG of patients with torsade de pointes (TdP) has been reported in methadone users. As heroin addicts sometimes faint while using illicit drugs, doctors might attribute too many episodes of syncope to illicit drug use and thereby underestimate the incidence of TdP in this special population, and the high mortality in this population may, in part, be caused by the proarrhythmic effect of methadone.
Methods
In this cross‐sectional study interview, ECGs and blood samples were collected in a population of adult heroin addicts treated with methadone or buprenorphine on a daily basis. Of the patients at the Drug Addiction Service in the municipal of Copenhagen, 450 (∼52%) were included. The QT interval was estimated from 12 lead ECGs. All participants were interviewed about any experience of syncope. The association between opioid dose and QT, and methadone dose and reporting of syncope was assessed using multivariate linear regression and logistic regression, respectively.
Results
Methadone dose was associated with longer QT interval of 0.140 ms/mg (p = 0.002). No association between buprenorphine and QTc was found. Among the subjects treated with methadone, 28% men and 32% women had prolonged QTc interval. None of the subjects treated with buprenorphine had QTc interval >0.440s½. A 50 mg higher methadone dose was associated with a 1.2 (95% CI 1.1 to 1.4) times higher odds for syncope.
Conclusions
Methadone is associated with QT prolongation and higher reporting of syncope in a population of heroin addicts.
QT prolongation and torsade de pointes (TdP) related to methadone treatment for heroin addiction has been described by several authors.1,2,3,4,5 The QT prolongation is caused by methadone blocking the HERG (human ether‐a‐go‐go related gene) potassium channels mimicking the type 2 of the long QT syndrome (LQT2).6 LQT2 is characterised by QT prolongation and flattened T waves, and associated with a higher risk of sudden cardiac death.7 At the same time, it is widely accepted and recommended that heroin‐dependent people should have access to methadone‐maintenance therapy.8 Although addicts treated with methadone have lower mortality rates than addicts who are not treated, the mortality rates are still much higher than that observed in a non‐addicted population.9 Many sudden deaths among these patients might be associated with a proarrhythmic effect of methadone.
Heroin addicts comprise a very vulnerable group of patients. The majority of the patients are unemployed and some are homeless. The widespread lack of compliance and mistrust of officials in this population make conventional research methods unfeasible. To confirm the low incidence of syncope, it would require a very large number of participants and long follow‐up time for studying the proarrhythmic effect of methadone. In the light of these difficulties, we chose a cross‐sectional design. This design precludes any direct proof of proarrhythmic effect, but makes it possible to evaluate associations between methadone dose and QT interval in the ECG in this special population.
The aims of our study were (1) to describe the association of the treatment with methadone and buprenorphine on the QT interval in the ECG and (2) to evaluate symptoms possibly related to arrhythmia in a large population of heroin addicts.
Methods
Patients
The Drug Addiction Service in the municipal of Copenhagen is a public service that offers free treatment for heroin addicts. Subjects were recruited from 10 different treatment centres. Inclusion criteria for our study were age >18 years and being in treatment with methadone or buprenorphine on a daily basis. All patients were diagnosed by World Health Organization ICD‐10 codes F11.2. Data collection was performed from July 2004 to February 2005.
Interview
The interview was performed as a standardised questionnaire concerning demographic data, misuse of specific drugs, symptoms of syncope, medical history considering heart disease and medical treatment. Because of a high prevalence of illiteracy among the participants, all questions were read aloud by a doctor who was not employed at the treatment programme. To get the most accurate information about misuse, the written consent stated that no information about the interview or ECG was shared with the staff of the treatment programme. We did not examine the validity of the self‐reported misuse. However, self‐reported misuse has been shown to be accurate under these conditions.10
The patients were asked if they had experienced any syncope in the past year. Syncope was defined as a sudden unexpected loss of consciousness without prior injection or inhalation of drugs.
ECG
A resting ECG of 10 s length was recorded just after the interview. All ECGs were recorded by the same doctor using the same 12‐lead ECG recorder (Corina, Marquette Hellige, Freiburg, Germany) between 9:00 and 15:00. The ECGs were recorded at trough level before the daily dose.
At the end of the collection of data, all ECGs were printed at paper speed 25 mm/s and amplification 10 mm/mV. The same doctor, blinded to patient data, assessed all ECG recordings.
The beginning of the QT interval was measured from the first deflection from the isoelectric line after the P wave. The ending of the QT interval was defined at the point where the steepest tangent at the descending part of the T wave crosses the isoelectric line. This is a simplification of Lagunas method.11 In the case of a bifid T wave, only T2 was considered as part of the T wave if the amplitude of T2 was similar or higher than the amplitude of T1.12 The longest QT interval measured in lead II, V2 or V5 from each ECG was used in the further analysis of data.
The adaptation of the QT interval to the heart rate is slow and the correction of the QT interval was done using the mean of all relative risk (RR) intervals in the ECG.13
The corrected QT interval was calculated using Bazett's formula14 and a derivate from Fridericia's formula15:
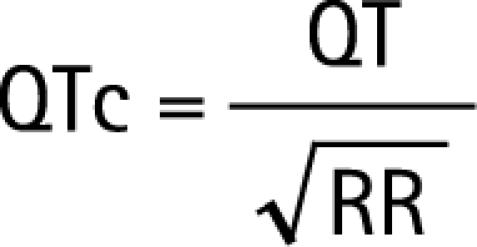 |
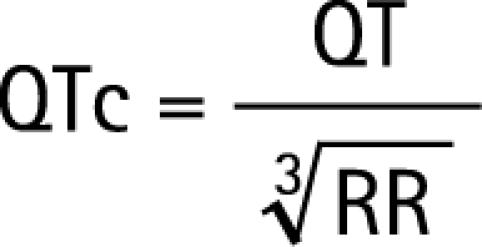 |
Patients having atrial fibrillation/flutter (n = 2), bundle branch block (n = 3), bigemini (n = 4) or pacemaker (n = 1) were excluded. Two patients receiving both methadone and buprenorphine were excluded. Further, two patients were excluded due to poor quality of the ECGs.
Blood sample
As most addicts are averse to giving blood samples, patients could be included in the study without giving blood sample. Blood sample was given by 54 of 450 participants. The blood sample was drawn in the minutes after the ECG. In the blood sample, s‐potassium was measured.
Statistical analysis
All variables are expressed as mean (SD). When data did not have normal distribution, median and percentiles were given, and non‐parametric methods were used. To evaluate the reproducibility of the QT measures, all ECGs were measured twice. We used the Bland and Altman method16 to assess agreement between the first and the second measure. The Bland–Altman plot (not shown) showed no relation between the difference and the mean of the two QT measurements. A total of 97.5% of all intervals were measured within ±0.02 s. This corresponds to ±0.5 mm at the standard ECG.
The association between methadone, age, duration of treatment and s‐potassium, and the QT interval was assessed by multivariate linear regression. The parameters in the multivariate regression were handled as continuous variables. The evaluation of the association between the frequency of symptoms and methadone dose was done by logistic regression. When logistic regression was performed, linearity was checked using Hosmer and Lemeshow goodness of fit test. All statistical calculations were done using SAS V.9.1. A p value <0.05 was considered significant.
Ethical considerations
The participants were physically and psychologically dependent on opioid, and studies including participants from among such patients have to consider the particular ethical situation. It is obvious that a patient who is dependent on a rather high dose of opioid might feel forced to participate when the prescribing doctor asks. We tried to avoid this problem by stating that no information from the interview or ECG was shared with the treatment system. The examining doctor was not employed at the treatment programme, and in the case of prolonged QT interval and symptoms of syncope, the doctor arranged further examination at a nearby hospital.
All patients gave informed consent and the local ethical review board approved the study ((KF)01‐075/04).
Results
In the city of Copenhagen, a total of 870 patients were in the treatment programme in the period of data collection: 450 (52%) of these patients were included in the study. The remaining 48% of the patients simply did not want to participate. There were no significant differences in average age (41.0 years among participants vs 41.1 years among non‐participants, p = 0.805) or sex distribution (female:male = 22.2% among participants vs female:male = 28.1% among non‐participants, p = 0.93).
Table 1 shows the demographic data of the included patients. A significant difference in age between participants under methadone treatment and under buprenorphine treatment was found. Participants under methadone treatment had been in the treatment programme longer than those under buprenorphine treatment. Misuse of cannabis was more frequent among participants under methadone treatment. There were no differences in the misuse of opioids, benzodiazepines and cocaine between the groups. Doses of buprenorphine and methadone were not normally distributed and percentiles are given instead of SD.
Table 1 Demographic data of the study patients.
| Patients treated with methadone | Patients treated with buprenorphine | p Value | |
|---|---|---|---|
| n | 407 | 43 | –– |
| Sex (F:M) | 107:300 | 9:34 | 0.4569 |
| Mean (SD) age (years) | 41.3 (8.3) | 38.0 (7.6) | 0.0145 |
| Treatment period (years) (median (10th and 90th percentile)) | 7 (0.5;20) | 1 (0.02;4) | <0.0001 |
| Methadone dose (mg) (median (10th and 90th percentile)) | 100 (50;235) | –– | –– |
| Buprenorphine dose (mg) (mean (10th and 90th percentile)) | –– | 5.4 (0.6;12) | –– |
| Patients with cocaine use past week | 76 (18.7%) | 5 (10.6%) | 0.2528 |
| Patients with illicit opioid use past week | 121 (29.7%) | 16 (34.0%) | 0.3108 |
| Patients with cannabis use past week | 243 (59.7%) | 19 (40.4 %) | 0.0497 |
| Patients with illicit benzodiazepine use | 88 (21.6%) | 5 (10.7%) | 0.1238 |
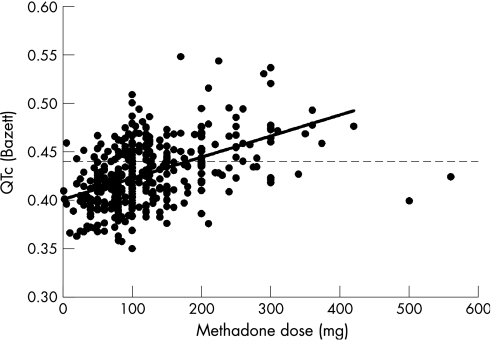
A significant association between QTc interval and methadone dose was found in both sexes. The association was present regardless of the correction formula used. Among men, the correlation coefficient was R2 = 0.28 (p<0.001) when QT was corrected using Bazett's formula and R2 = 0.26 (p<0.001) when Fridericia's formula was used. Among women, the correlation coefficient was R2 = 0.12 (p<0.001) when QT was corrected using Bazett's formula and R2 = 0.17 (p<0.001) when Fridericia's formula was used. Figure 1 shows the relation between oral methadone dose and QTc among all participants treated with methadone (n = 393). In all, 127 (32%) participants treated with methadone had QTc>0.440s½.
Figure 1 The scatter plot shows the corrected QT interval plotted against the methadone dose among all participants treated with methadone (n = 393). The regression line was interpolated without using two outliers at 500 and 560 mg. The dotted horizontal line corresponds to the upper limit of the normal range of the QTc.
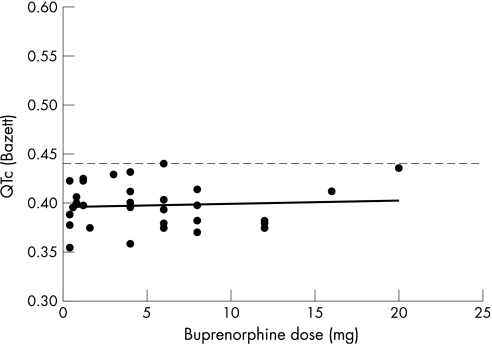
There was no association between QTc interval and buprenorphine dose. Figure 2 shows the relation between oral buprenorphine dose and QTc. None of the 9 women and 34 men treated with buprenorphine had QTc >0.440s½.
Figure 2 The scatter plot shows the corrected QT interval plotted against the buprenorphine dose (n = 43). The dotted line corresponds to the upper limit of the normal range. None of the participants treated with buprenorphine had QTc above the upper limit of the normal range.
To estimate the relationship between QT interval and RR interval, methadone dose, s‐potassium, duration of treatment and age, multivariate linear regression analysis was done. Methadone dose was significantly associated with QT prolongation; s‐potassium was associated with a significant QT shortening. We did not find any significant association between age or duration of treatment and the QT interval. Table 2 shows the parameter estimates from the multivariate linear regression.
Table 2 Parameter estimates from the multivariate linear regression.
| Variable | Parameter estimate | p Value | 95% CI |
|---|---|---|---|
| Intercept (ms) | 329 | <0.001 | 228 to 430 |
| Age (ms/year) | 0.03 | 0.9546 | −1.1 to 1.2 |
| Duration of treatment (ms/year) | 0.88 | 0.2288 | −0.57 to 2.32 |
| Methadone dose (ms/mg) | 0.14 | 0.0017 | 0.06 to 0.23 |
| S‐K (ms/mM) | −27.5 | 0.0153 | −49.5 to −5.49 |
| RR | 177 | <0.001 | 139 to 215 |
ms, millisecond; S‐K, s‐potassium.
Syncope reporting
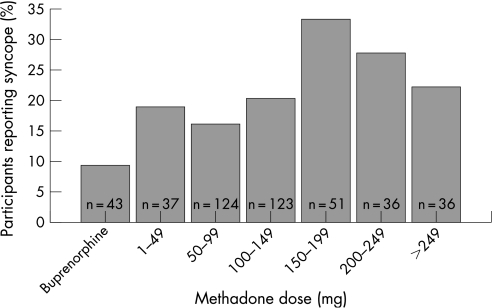
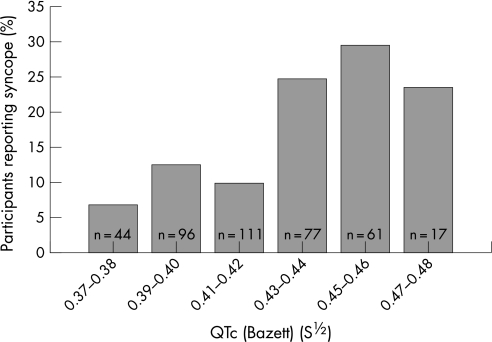
To evaluate the association between methadone dose and syncope during the past year, a logistic regression analysis was used. Odds for reporting any syncope were 1.2 (95% CI 1.1 to 1.4) times higher when methadone dose was increased by 50 mg. Figure 3 shows the percentage of participants having reported syncope during the past year. In fig 3, participants are categorised in dose intervals of 50 mg according to methadone dose. The proportion of participants reporting syncope was higher at higher methadone dose and was lowest in the group treated with buprenorphine. The association between QTc and reporting of syncope during the past year was evaluated using logistic regression. A 0.01s½ longer QTc was associated with a 1.11 (95% CI 1.04 to 1.20) times higher odds for reporting syncope during the past year. Figure 4 shows the percentage of participants reporting syncope according to their QTc interval.
Figure 3 The percentage of participants reporting syncope in different dose categories. The numbers in the bars show the number of participants in the corresponding dose category. The lowest percentage of participants reporting syncope was found in the group treated with buprenorphine.
Figure 4 The percentage of participants reporting syncope according to their QTc interval. The numbers in the bars show the number of participants in the corresponding QTc interval category.
Misuse of illicit opioid
Logistic regression was used to estimate the association between methadone dose and odds for reporting misuse. Odds for reporting misuse were 0.7 (95% CI 0.55 to 0.90) times and 0.6 (95% CI 0.36 to 0.95) times lower when methadone dose was increased by 50 mg among men and women, respectively. Among subjects at methadone dose >250 mg, only 2 of the 36 participants reported any misuse of opioids during the past week.
Discussion
To our knowledge, this is the first study to investigate reporting of syncope in a population treated for heroin dependence. Syncope is difficult to deal with as it is very rare and a large number of patients are required to measure the incidence. In this special population, syncope can be caused by many factors—for example, use of heroin and other drugs. Alternating use of benzodiazepines can also lead to epileptic convulsions and syncope.
We have found an increasing incidence of syncope with higher doses of methadone. This could be explained by more excessive misuse of opioids by patients at a higher methadone dose. According to the self‐reported misuse of opioids during the past week, this is probably not the case. We found that misuse of opioid decreases with higher doses of methadone. Another explanation could be that methadone causes QT prolongation, which increases the incidence of TdP. Even though the direct evidence cannot be provided from this study, this explanation seems plausible as it has been shown that methadone can block HERG‐channel,6 and blockade of these channels causes QT and QTc prolongation that can lead to TdP.17 In this population, we also found that a longer QTc is associated with higher odds for the reporting of syncope. Other studies have shown an association between the prolongation of QT/QTc interval and sudden death in populations without evidence of cardiac dysfunction.18,19 Potassium‐channel blockade could also be associated with syncope without TdP. A small study indicated that patients with genetic defects in potassium channels might faint due to neurocardiogenic reasons.20 Autonomic neuropathy could also account for syncope in this population. Villa et al21 have shown that autonomic neuropathy is present in a population which mostly comprised of drug users. According to Cencetti et al,22 autonomic neuropathy can affect the control of cerebral circulation and thereby cause syncope not related to cardiac arrhythmias.
In this study, we also found a significant association between methadone dose and QT and QTc interval. This finding is in agreement with Martell et al,23 who, in a prospective study, found a significant QTc prolongation during 6‐ and 12‐months treatment with methadone. Similarly, Kornick et al24 showed a dose‐dependent QTc prolonging effect of intravenous methadone compared with intravenous morphine. In a retrospective study, Ehret et al25 have also found an association between methadone dose and QTc interval. This study also showed that discontinuation of methadone was associated with shorter QTc interval. However, Maremmani et al26 did not find any correlation between oral methadone dosage and QTc, although they found that patients on long‐term methadone maintenance therapy had longer QT interval compared with people not on long‐term methadone of the same sex and age. The reason that Maremmani et al did not find a correlation might be the measuring technique applied by them. Measuring QT intervals is not easy especially when U waves are present. In this study, U waves are not included in the QT interval. Martell et al also describe strict criteria for including the U wave in the QT interval. Maremmani et al do not describe their technique regarding the inclusion of the U wave or whether the QT intervals are measured manually or automatically.
In the multivariate linear regression of the data from participants who gave blood sample, it was found that the QT lengthening effect of 200 mg methadone was roughly equivalent to that of a l mM lower s‐potassium. The duration of the methadone‐maintenance therapy was not associated with the length of the QT interval. The fact that the current dose rather than cumulated dose affects the QT interval indicates a purely reversible mechanism such as temporary blocking of ion channels. This supports Katchman et al6 who did voltage‐clamp recordings on cells expressing the HERG gene which codes for the ion channel leading the Ikr current. These voltage‐clamp experiments showed that methadone blocks the HERG channels, mimicking the molecular defect in LQT2. This is also in agreement with Ehret et al25 who showed that discontinuation of methadone was associated with shorter QTc interval.
This study is the first to investigate the association between buprenorphine and the QT interval in a group of patients treated with buprenorphine. The buprenorphine group was much smaller than the methadone group, but it corresponds to the proportion of patients treated with buprenorphine in the total population of heroin dependents in the city of Copenhagen. The patients treated with buprenorphine were on an average 3.2 years younger than the patients treated with methadone. Despite the differences between the two groups, we believe that this group is a better control group than a normal population. We did not find any patient with prolonged QT interval in the group treated with buprenorphine and we were not able to find any correlation between buprenorphine and QT interval or QTc. Buprenorphine is known to block HERG in vitro, but only at concentrations much higher than those found in patients treated with buprenorphine. Katchman et al6 found that the IC50:Cmax ratio for buprenorphine was 208, whereas it was only 2.7 for methadone.
The fact that the patients treated with buprenorphine only received low doses made us cautious in excluding a QT prolonging effect of buprenorphine at higher doses. However, Krantz et al27 have reported that a patient with methadone‐related TdP was successfully treated with buprenorphine instead of methadone.
Implications
Estimating the exact risk for TdP from a QT interval is probably not possible. However, the fact that every new drug now has to undergo a QT study before registration reflects the need to understand the propensity of the drug to prolong the QT interval and thereby optimise their use. ICH E‐14 (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) is a new guidance for clinical evaluation of prolongation of the QT and/or QTc interval and proarrhythmic potential for non‐antiarrhythmic drugs. The ICH E‐14 states that there is no consensus regarding the choice of upper limit values of the QT interval and changes from baseline. However, the risk of TdP usually begins at QTc about 0.500 s and exponentially rises thereafter.28 Only eight of the participants in this study had QT or QTc >0.500 s. These participants received >100 mg methadone, and therefore we suggest that ECG recording is done when methadone dose surpass this limit. If QT or QTc is above the normal range, the indication for methadone should be carefully re‐evaluated and if QT or QTc exceeds 0.500 s, methadone treatment should be discontinued. Patients with substantial QT prolongation should be switched to buprenorphine.
As the QT prolonging effect of two concomitant HERG blocking drugs is believed to be synergistic, it is very important to avoid concomitant use of other drugs capable of blocking HERG channels.
Use of drugs causing hypokalaemia should also be avoided, as hypokalaemia can modulate Ikr and thus cause QT prolongation.29,30
Limitations
It is not possible to show any causal connections from a cross‐sectional study and therefore we are only able to describe the incidence of syncope in the population. It would require a prospective study with continuous monitoring to deliver the direct evidence that the syncopes are caused by arrhythmia.
Syncopes are rare even in this population and most syncopes are not recent ones. As the misuse measure in this study only relates to misuse during the past week, there is no evidence that the participants had a similar pattern of misuse at the time of their syncopes. Consequently, we cannot rule out the fact that misuse causes syncope.
Conclusion
Treatment with methadone in a population of heroin dependents is associated with QT and QTc prolongation. No QT or QTc prolongations were found in a similar population treated with buprenorphine.
Patients on a higher methadone dose reported higher incidence of syncope even though the misuse of illicit opioids was lower. The direct evidence that methadone causes the QTc prolongation and thereby increases the incidence of syncope cannot be provided from an epidemiological study.
Acknowledgements
The Danish Ministry of the Interior and Health provided a grant.
Abbreviations
HERG - human ether‐a‐go‐go related gene
LQT2 - type 2 of the long QT syndrome
TdP - torsade de pointes
Footnotes
Competing interests: None.
References
- 1.Krantz M J, Lewkowiez L, Hays H.et al Torsade de pointes associated with very‐high‐dose methadone. Ann Intern Med 2002137501–504. [DOI] [PubMed] [Google Scholar]
- 2.Hassan J, Bent‐Hansen L, Jensen G. Livstruende, repetitiv arytmi hos patienter i højdosis methadonbehandling: torsade de pointes. Ugeskr Laeger 20041663104–3105. [PubMed] [Google Scholar]
- 3.Walker P W, Klein D, Kasza L. High dose methadone and ventricular arrhythmias: a report of three cases. Pain 2002103321–324. [DOI] [PubMed] [Google Scholar]
- 4.Walker G, Wilcock A, Carey A M.et al Prolongation of the QT interval in palliative care patients. J Pain Symptom Manage 200326855–859. [DOI] [PubMed] [Google Scholar]
- 5.Gil M, Sala M, Anguera I.et al Qt prolongation and torsades de pointes in patients infected with human immunodeficiency virus and treated with methadone. Am J Cardiol 200392995–997. [DOI] [PubMed] [Google Scholar]
- 6.Katchman A N, Mcgroary K A, Kilborn M J.et al Influence of opioid agonists on cardiac human ether‐a‐go‐go‐related gene K currents. J Pharmacol Exp Ther 2002303688–694. [DOI] [PubMed] [Google Scholar]
- 7.Kanters J K, Fanoe S, Larsen L A.et al T wave morphology analysis distinguishes between KvLQT1 and HERG mutations in long QT syndrome. Heart Rhythm 20041285–292. [DOI] [PubMed] [Google Scholar]
- 8.National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction Effective medical treatment of opiate addiction. JAMA 19982801936–1943. [PubMed] [Google Scholar]
- 9.Joseph H, Stancliff S, Langrod J. Methadone maintenance treatment (MMT): a review of historical and clinical issues. Mt Sinai J Med 200067347–364. [PubMed] [Google Scholar]
- 10.Langendam M W, van Haastrecht H J, van Ameijden E J. The validity of drug users' self‐reports in a non‐treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int J Epidemiol 199928514–520. [DOI] [PubMed] [Google Scholar]
- 11.Laguna P, Thakor N V, Caminal P.et al New algorithm for QT interval analysis in 24‐hour Holter ECG: performance and applications. Med Biol Eng Comput 19902867–73. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg Ilan, Moss AJ, Zareba Wojc QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol 200617333–336. [DOI] [PubMed] [Google Scholar]
- 13.Franz M R, Swerdlow C D, Liem L.et al Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady‐state frequencies. J Clin Invest 198882972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazett H C. An analysis of the time‐relations of electrocardiograms. Heart 19207353–370. [Google Scholar]
- 15.Fridericia L. Die systolendauer im Elektrokardiogramm bei normalen Menschen und bei herzkranken. Acta Med Scand 192053469–486. [Google Scholar]
- 16.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986327307–310. [PubMed] [Google Scholar]
- 17.Shimizu W, Antzelevitch C. Cellular basis for long QT, transmural dispersion of repolarization, and torsade de pointes in the long QT syndrome. J Electrocardiol 199932177–184. [DOI] [PubMed] [Google Scholar]
- 18.Algra A, Tijssen J G, Roelandt J R.et al QTc prolongation measured by standard 12‐lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 1991831888–1894. [DOI] [PubMed] [Google Scholar]
- 19.Elming H, Holm E, Jun L.et al The prognostic value of the QT interval and QT interval dispersion in all‐cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J 1998191391–1400. [DOI] [PubMed] [Google Scholar]
- 20.Toft E, Aaroe J, Jensen B T.et al Long QT syndrome patients may faint due to neurocardiogenic syncope. Europace 20035367–370. [DOI] [PubMed] [Google Scholar]
- 21.Villa A, Foresti V, Confalonieri F. Autonomic neuropathy and prolongation of QT interval in human immunodeficiency virus infection. Clin Auton Res 1995548–52. [DOI] [PubMed] [Google Scholar]
- 22.Cencetti S, Lagi A, Cipriani M.et al Autonomic control of the cerebral circulation during normal and impaired peripheral circulatory control. Heart 199982365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martell B A, Arnsten J H, Krantz M J.et al Impact of methadone treatment on cardiac repolarization and conduction in opioid users. Am J Cardiol 200595915–918. [DOI] [PubMed] [Google Scholar]
- 24.Kornick C A, Kilborn M J, Santiago‐Palma J.et al QTc interval prolongation associated with intravenous methadone. Pain 2003105499–506. [DOI] [PubMed] [Google Scholar]
- 25.Ehret G B, Voide C, Gex‐Fabry M.et al Drug‐induced long QT syndrome in injection drug users receiving methadone: high frequency in hospitalized patients and risk factors. Arch Intern Med 20061661280–1287. [DOI] [PubMed] [Google Scholar]
- 26.Maremmani I, Pacini M, Cesaroni C.et al QTc interval prolongation in patients on long‐term methadone maintenance therapy. Eur Addict Res 20051144–49. [DOI] [PubMed] [Google Scholar]
- 27.Krantz M J, Garcia J, Mehler P. Effects of buprenorphine on cardiac repolarization in a patient with methadone‐related torsade de pointes. Pharmacotherapy 200525611–614. [DOI] [PubMed] [Google Scholar]
- 28.Shah R R. Pharmacogenetic aspects of drug‐induced torsade de pointes: potential tool for improving clinical drug development and prescribing. Drug Saf 200427145–172. [DOI] [PubMed] [Google Scholar]
- 29.Sanguinetti M C, Jiang C, Curran M E.et al A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 199581299–307. [DOI] [PubMed] [Google Scholar]
- 30.Yan G ‐ X, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT syndrome. Circulation 1998981928–1936. [DOI] [PubMed] [Google Scholar]