Abstract
Background
Circulating endothelial progenitor cells (EPCs) are increased in conditions associated with ischaemia and can potentially support angiogenesis and vasculogenesis. EPC levels were also shown to predict outcome in patients with atherosclerotic vascular disease. We tested the hypothesis that circulating EPC can predict outcome in patients with congestive heart failure (CHF).
Methods
EPC–colony‐forming units were measured in the peripheral blood of 107 consecutive patients with CHF with New York Heart Association (NYHA) functional class II–IV. Serum levels of vascular endothelial growth factor (VEGF), N‐terminal pro‐B‐type natriuretic peptide (NT‐pro‐BNP) and high‐sensitivity C‐reactive protein (hsCRP) were also measured. End points were defined as CHF‐related hospital admissions and all‐cause mortality.
Results
Age (p = 0.01), diabetes mellitus (p = 0.002) and EPC levels (p = 0.02) were found to be independent predictors of all‐cause mortality. EPC levels did not predict CHF‐related hospitalisations. EPC levels correlated positively with NYHA (p = 0.05, r = 0.19), but did not correlate with VEGF, NT‐pro‐BNP or hsCRP. EPC levels did not differ by the aetiology of CHF.
Conclusions
EPC levels are independent predictors of all‐cause mortality among patients with CHF.
Recent population‐based studies on patients with heart failure (HF)1,2 report an improved survival and a trend towards a plateau or a decline in hospital admissions. These encouraging reports are attributed to improved understanding of disease mechanisms, and to the development of new pharmacological and mechanical treatment modalities. However, despite improved outcome, HF continues to be a prevalent disease with substantial morbidity and mortality.
In an attempt to improve patient care, clinicians try to define clinical and/or laboratory markers of an adverse outcome. Clinical factors include high New York Heart Association (NYHA) functional class, low left ventricular ejection fraction (LVEF), advanced age, cachexia and related comorbidities including renal disease, anaemia and diabetes mellitus.1,3,4 Laboratory markers include mediators of the neurohormonal and inflammatory axes that are activated in these patients. In this respect, B‐type natriuretic peptide (BNP),5 and to a lesser extent C‐reactive protein,6 were shown to predict clinical outcome in patients with congestive heart failure (CHF). None of these clinical or laboratory markers is either sensitive or specific enough, and development of additional predictors is needed.
Endothelial progenitor cells (EPCs)7 reside in the bone marrow and are found to some extent in the peripheral blood. EPCs participate in vasculogenesis through their capacity to proliferate, migrate and differentiate, and serve as paracrine factories for proangiogenic cytokines.7 Conditions that induce tissue ischaemia or endothelial damage—that is, vascular trauma,8 acute myocardial infarction9 and unstable angina10—are associated with a release of angiogenic factors, including vascular endothelial growth factor (VEGF) and erythropoietin, which promote mobilisation of EPC from the bone marrow to the peripheral circulation. A decrease in EPC levels was shown to correlate inversely with atherosclerotic risk factors11 and the occurrence of ischaemic stroke,12 and to correlate with endothelial dysfunction,11,13 accelerated atherosclerosis14 and in‐stent restenosis.15
CHF is characterised by tissue ischaemia and endothelial dysfunction.3 Indeed, it has recently been demonstrated that patients with CHF have elevated circulating EPC levels16 compared with healthy controls. However, whether this observation has prognostic clinical implications has not yet been resolved.
In this study, we have made a quantitative assessment of circulating EPC in patients with CHF and studied their predictive value on clinical outcome including mortality and CHF‐related hospital admissions.
Methods
Patients
The study included 107 consecutive patients with CHF attending the outpatient clinic of the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, between September 2003 and March 2004. This is a referral clinic: all patients attending which have had symptomatic HF and had undergone an echocardiographic examination. Patients with chronic HF in NYHA II–IV were recruited. The clinical diagnosis of HF was based on the history of acute pulmonary oedema or two of the following signs or symptoms not explained by other identifiable causes and improved with diuresis: exertional dyspnoea or fatigue, paroxysmal nocturnal dyspnoea, orthopnoea, pleural effusion or bilateral lower extremity oedema. Patients with LVEF >45% and echocardiographic signs of diastolic dysfunction were diagnosed as having HF with preserved systolic function. LVEF was measured echocardiographically. Echocardiographic criteria for diastolic dysfunction were mainly based on mitral valve inflow pattern as described elsewhere17 and in some patients in conjunction with pulmonary vein flow pattern and mitral annular Doppler tissue imaging. Patients were examined, and they answered a thorough questionnaire on medical history, performance status, atherosclerotic risk factors and medications. Patients were followed every 3 months or at higher frequency as dictated by the patients' clinical status.
Follow‐up
The study end points were all‐cause mortality and hospital admission due to HF.
End points data were collected during follow‐up visits. In addition, records from hospital admissions were reviewed to confirm whether it was CHF related. No patient was lost to follow‐up that ended on January 2005.
The local ethics committee approved the study.
Isolation of EPC and colony‐forming unit assay
At baseline, 20 ml of blood was withdrawn for the isolation of EPC as described previously.11,12 Briefly, peripheral blood mononuclear cells were isolated by Ficoll density‐gradient centrifugation (Sigma, St. Louis, Maryland, USA). After washing, isolated cells were resuspended in growth medium and plated on dishes coated with human fibronectin (Chemicon, Temecula, California, USA). To eliminate initial contamination with mature circulating endothelial cells, preplating of peripheral blood mononuclear cells onto fibronectin‐coated six well plates was performed (5×106/well) for 48 h, after which non‐adherent cells were collected and replated onto fibronectin‐coated plates for a final evaluation of colony numbers counted at day 7.
Confirmation of colony‐forming unit phenotype
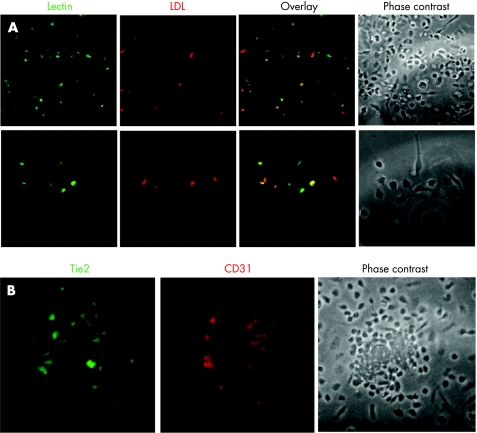
Colonies were assayed for endothelial cell markers at day 7. The following antibodies were used for immunofluorescentic and flow‐cytometric phenotyping: rabbit polyclonal anti‐Tie‐2 (C‐20), mouse monoclonal anti‐flk‐1 (A‐3) and goat polyclonal anti‐CD31 (platelet endothelial cell adhesion molecule 1, M‐20), all from Santa‐Cruz. Endothelial‐cell lineage was further confirmed by indirect immunostaining with 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate‐acetylated low‐density lipoprotein and co‐staining with bandeiraea simplicifolia ‐1 lectin (both from Sigma).
ELISA for the detection of VEGF
Quantitative determination of VEGF was performed using sandwich ELISA kit according to the manufacturer's protocol (R&D Systems, Minneapolis, Minnesota, USA).
High‐sensitivity C‐reactive protein concentrations
High‐sensitivity C‐reactive protein (hsCRP) concentration was determined by the Behring BN II Nephelometer according to the manufacturer's instructions (Dade Behring, Deerfield, Illinois, USA).
Determination of N‐terminal pro‐BNP levels
Measurements of serum N‐terminal pro‐B‐type natriuretic peptide (NT‐pro‐BNP) were done by automated immunoassay (Elecsys proBNP, Roche Diagnostics, Mannheim, Germany). The test principle included using two polyclonal antibodies directed against NT‐pro‐BNP—epitope 1: amino acid 1–21 and epitope 2: amino acid 39–50. The results were calibrated against a synthetic NT‐pro‐BNP (amino acid 1–76). The range of results was between 5 and 35 000 pg/ml.
Statistical analysis
End point analysis based on Cox proportional‐hazards model was used to analyse the data. A different model was constructed for each of the following adverse events: mortality and CHF‐related hospital admissions. These models explain the independent relationship between each parameter and time to incidence, and enables us to estimate the relative hazard ratio (HR) for each risk factor. Variables generally accepted as being of prognostic value in CHF were inserted, including age, gender, NYHA, LVEF, smoking, diabetes mellitus, hypertension, hyperlipidaemia, history of ischaemic heart disease, and laboratory parameters of proBNP, hsCRP, VEGF and EPC level.
Comparison regarding EPC levels between two or more groups was performed by Student's t test and analysis of variance. Correlations were examined by Pearson's correlation test.
The statistical significance level was set to 0.05 and SPSS V.12.0 was used for the analysis.
Results
Table 1 shows the baseline clinical characteristics of our cohort. The aetiology for CHF was ischaemic heart disease in 74 (69.1%) patients, idiopathic dilated cardiomyopathy in 12 (11.2%), valvular disease in 11 (10.2%) and hypertensive disease in 10 (9.3%) patients. In all, 78 (72.8%) patients had systolic HF and 29 (27.1%) had HF with preserved systolic function. NYHA distribution was as follows: 38 (35.5%) patients in class II, 61 (57%) in class III and 8 (7.4%) in class IV. Patients were followed for a minimum of 10 months and for a maximum of up to 17 months, mean 13.8 (1.96). During follow up, there were 21 (19.6%) mortality events, 2 of them were non‐cardiac deaths, and 26 (24.2%) patients were hospitalised due to exacerbation of CHF. Table 2 presents the levels of the different tested parameters in patients with and without the event. As it shows, EPC levels were highest among patients with mortality events.
Table 1 Clinical characteristics of the outpatient cohort with congestive heart failure (n = 107).
| Age, years* | 71.3 (10.1) |
| Males (%) | 84 (78.5) |
| HF duration, years† | 3.2 (1.7) |
| NYHA* | 2.6 (0.5) |
| LVEF* | 35.3% (14.1%) |
| Hyperlipidaemia | 65 (60.7) |
| Smoking | 10 (9.3) |
| Past smoker | 64 (59.8) |
| Hypertension | 73 (68) |
| Diabetes mellitus | 47 (43) |
| Ischaemic heart disease | 79 (73.8) |
| Chronic atrial fibrillation | 12 (11.2) |
| Transient ischaemic attacks/cerbrovascular accidents | 8 (7.4) |
| Percutaneous transluminal coronary angioplasty | 64 (59.8) |
| Coronary artery bypass surgery | 45 (42) |
| ACE inhibitors/ARBs | 81 (75.7) |
| Spironolactone | 53 (49.3) |
| β blockers | 66 (61.6) |
| Statins | 65 (60.7) |
| Digoxin | 21 (19.6) |
| Diuretics | 104 (97) |
| Anticoagulants | 32 (29.9) |
ARB, angiotensinogen receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
*Values are given as mean (SD).
All other values are mean (%).
†Disease duration before enrolment.
Table 2 Levels of the different tested parameters in patients with and without the event.
| Event* | All patients | Mortality | Hospital admission | No events |
|---|---|---|---|---|
| n = 107 | n = 21 | n = 26 | n = 70 | |
| Variable | ||||
| EPC–CFU | 81.08 (67.5) | 98.48 (81.84) | 71.15 (58.55) | 77.29 (62.46) |
| NT‐pro‐BNP, pg/ml | 1942 (2626) | 3622 (7333) | 3784 (6587) | 1732 (2994) |
| hsCRP, μg/ml | 10.66 (26.59) | 14.95 (24.89) | 13.65 (19.52) | 8.68 (27.99) |
| VEGF, pg/ml | 69.53 (71.18) | 66.35 (41.65) | 77 (17.8) | 67.7 (50) |
EPC–CFU, endothelial progenitor cells–colony‐forming units; hsCRP, high‐sensitivity C‐reactive protein; NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide; VEGF, vascular endothelial growth factor.
*10 patients had CHF‐related hospital admission prior to mortality event.
Values are given as mean (SD).
A representative example of EPC–colony‐forming unit (CFU) and its phenotype confirmation is depicted in fig 1.
Figure 1 Confirmation of endothelial progenitor cell (EPC) phenotype by immunohistochemistry. To validate the identity of EPC, we employed two methods as described in Methods. (A) Confirmation of adherent EPC by costaining with anti‐acetylated low‐density lipoprotein (LDL) and anti‐bandeiraea simplicifolia ‐1. (B) Endothelial marker expression of the colony‐forming unit counted by antibodies to Tie‐2 and CD‐31.
There were no statistically significant differences in the levels of EPC–CFU between patients with systolic as compared with diastolic HF (78.4 (60.9) and, 87.4 (82.1)/well, respectively, p = 0.71). EPC–CFU levels did not correlate with the aetiology of CHF. EPC–CFU numbers in ischaemic HF, idiopathic dilated cardiomyopathy, and valvular and hypertension related HF were 79.3 (69.9), 79.6 (61.4), 102.2 (63.9) and 71 (67.8)/well, respectively (p = NS for all comparisons). There was no correlation between EPC–CFU levels and circulating VEGF (p = 0.31), proBNP (p = 0.92) or hsCRP (p = 0.13). However, a statistically significant correlation was found with NYHA (p = 0.05, r = 0.188). EPC levels did not correlate with disease duration.
In order to define the clinical relevance of EPC levels to future outcomes, a Cox regression model was used. Table 3 provides multiple adjusted HRs of the clinical and laboratory parameters that were examined as predictors of mortality. Advanced age, diabetes mellitus and levels of EPC–CFU were the only independent variables predictive of mortality in our study cohort. Severely compromised LVEF trended as an independent predictor. Examining the same model on cardiac death alone yielded the same results (p values: age, 0.01; diabetes mellitus, 0.003; EPC, 0.02; and LVEF, 0.058). Table 4 provides data on CHF‐related hospital admissions. Only female gender and NT‐pro‐BNP levels were independent predictors of CHF‐related hospital admissions. Table 5 gives data regarding mortality prediction among patients with systolic HF. None of the parameters tested were statistically significant among patients with HF and preserved systolic function.
Table 3 Cox regression model: variables as predictors of mortality.
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Age, years | 1.097 (1.019 to 1.182) | 0.01 |
| Female gender | 0.599 (0.158 to 2.265) | 0.45 |
| NYHA | 1.035 (0.295 to 3.64) | 0.95 |
| LVEF | 0.966 (0.929 to 1.005) | 0.08 |
| Hyperlipidaemia | 0.593 (0.189 to 1.857) | 0.37 |
| Smoking | 0.662 (0.203 to 2.162) | 0.49 |
| Hypertension | 0.457 (0.127 to 1.651) | 0.23 |
| DM | 6.276 (1.972 to 19.969) | 0.002 |
| IHD | 1.135 (0.263 to 4.888) | 0.86 |
| EPC | 1.008 (1.001 to 1.015) | 0.02 |
| NT‐pro‐BNP | 1.043 (0.952 to 1.143) | 0.36 |
| hsCRP | 1.006 (0.991 to 1.02) | 0.45 |
| VEGF | 1.001 (0.988 to 1.014) | 0.87 |
DM, diabetes mellitus; EPC, endothelial progenitor cell; hsCRP, high‐sensitivity C‐reactive protein; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; VEGF, vascular endothelial growth factor.
Table 4 Cox regression model: variables as predictors of CHF‐related hospital admissions.
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Age, years | 0.981 (0.937 to 1.027) | 0.41 |
| Female gender | 3.433 (1.153 to 10.222) | 0.02 |
| NYHA | 1.629 (0.658 to 4.029) | 0.29 |
| LVEF | 0.978 (0.946 to 1.01) | 0.17 |
| Hyperlipidaemia | 1.097 (0.436 to 2.76) | 0.84 |
| Smoking | 0.825 (0.29 to 2.353) | 0.72 |
| Hypertension | 1.629 (0.593 to 4.472) | 0.34 |
| DM | 1.027 (0.426 to 2.476) | 0.95 |
| IHD | 1.055 (0.372 to 2.995) | 0.91 |
| EPC | 0.996 (0.989 to 1.004) | 0.31 |
| NT‐pro‐BNP | 1.069 (1.004 to 1.139) | 0.03 |
| hsCRP | 1 (0.987 to 1.014) | 0.94 |
| VEGF | 1.001 (0.995 to 1.007) | 0.79 |
DM, diabetes mellitus; EPC, endothelial progenitor cell; hsCRP, high‐sensitivity C‐reactive protein; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; VEGF, vascular endothelial growth factor.
Table 5 Cox regression model: variables as predictors of mortality among patients with systolic heart failure (n = 78).
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Age | 1.17 (1.023 to 1.338) | 0.02 |
| Female gender | 0.674 (0.104 to 4.366) | 0.67 |
| NYHA | 0.233 (0.035 to 1.573) | 0.13 |
| LVEF | 0.844 (0.754 to 0.944) | 0.003 |
| Hyperlipidemia | 1.176 (0.184 to 7.521) | 0.86 |
| Smoking | 3.033 (0.356 to 25.83) | 0.31 |
| Hypertension | 1.623 (0.221 to 11.939) | 0.63 |
| DM | 12.647 (1.778 to 89.966) | 0.01 |
| IHD | 0.457 (0.063 to 3.297) | 0.43 |
| EPC | 1.019 (1.002 to 1.036) | 0.02 |
| NT‐pro‐BNP | 1.16 (1.042 to 1.291) | 0.006 |
| hsCRP | 0.995 (0.968 to 1.023) | 0.71 |
| VEGF | 1.015 (0.998 to 1.033 | 0.07 |
DM, diabetes mellitus; EPC, endothelial progenitor cell; hsCRP, high‐sensitivity C‐reactive protein; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; VEGF, vascular endothelial growth factor.
Discussion
The present study is the first to show that levels of circulating EPC are an independent predictor of all‐cause mortality in patients with CHF. In fact, in our cohort, three independent variables were found to independently predict mortality, including advanced age, diabetes mellitus and EPC–CFU levels.
The study cohort included patients with advanced CHF selected from the outpatient clinic who were all controlled and were not admitted to hospital during the past 6 months so as not to introduce bias from potential exacerbation of their primary disorder. It should be noted that EPC levels failed to predict CHF‐related hospital admissions. This can be explained partially by the relatively high mortality of this cohort of patients.
Subgroup analysis demonstrated that the value of EPC levels as mortality predictors was limited to patients with systolic HF. Whether this is related to the smaller cohort of patients with preserved systolic function, or reflects inferiority of the predictive value among this patients' population needs further investigation.
There are several potential explanations for the predictive power of circulating EPCs regarding mortality. HF is characterised by peripheral and myocardial tissue ischaemia. This in turn results in systemic release of angiogenic factors and activation of multiple neurohormonal axes that may promote the peripheral mobilisation of EPCs from the bone marrow niche in an attempt to facilitate a process of angiogenesis and vasculogenesis.7 The more severe the ischaemia, the higher the driving force for the release of EPCs. Additionally, patients with CHF are known to exhibit endothelial dysfunction that is correlated with disease severity.3 Several studies have demonstrated that circulating EPCs serve as a pool of progenitor cells11,18 that participate in endothelial repair processes; thus, a larger pool may reflect a higher need for progenitor cells due to more intense endothelial damage.
Studies on animals have suggested that bone‐marrow‐derived progenitor cells may transdifferentiate into myocardial and vascular cells after myocardial injury, as an attempt to limit the extent of myocardial damage.19,20,21 It is tempting to speculate that these mechanisms are also operative in patients with HF, and that EPC mobilisation is a compensatory response intended to attenuate cardiac remodelling.
Valgimigli et al16 recently tested EPC levels in patients with CHF. They found that as a group, patients with CHF had higher EPC levels than healthy controls. However, they reported a bimodal behaviour of EPC levels, with higher levels in early stages of CHF and lower levels in advanced stages of CHF. They proposed a mechanism of bone marrow exhaustion by cytokines such as tumour necrosis factor α that are increased in advanced CHF. These results are in contrast with our findings of a correlation between EPC levels and NYHA. The difference may be partially explained by the different patient population studied—that is, in our cohort, 27% of patients had HF with preserved systolic function, and we included patients with NYHA II–IV, with only a minority from NYHA IV, whereas in the cohort studied by Valgimigli et al16 all patients had systolic HF and were evenly distributed from functional class I to IV. Our patients had more advanced CHF, but all were well controlled in a dedicated HF clinic. Moreover, statins, that were shown to influence EPC levels22 were discontinued only in that study. It should be noted that the study by Valgimigli et al16 was observational and was not designed to test the prognostic value of EPC.
In accordance with Valgimigli et al,16 we found that EPC levels were not influenced by the aetiology of CHF and did not correlate with VEGF, hsCRP or NT‐pro‐BNP. The finding in our study that EPC levels correlated with NYHA may imply that the clinical state of the patient is the dominant factor that influences EPC levels, although the exact mechanism is probably complex and multifactorial.
Similar to previous reports, diabetes mellitus was found to be an independent predictor of mortality.3 EPC in patients with diabetes has impaired functions including proliferation, adhesion and tubulisation.23 These functions are needed for the amplification of EPC, the attachment to the area of damaged endothelium and the creation of new blood vessels. EPC malfunction may thus contribute to the adverse prognosis of patients with diabetes. Future studies regarding EPC function in CHF and its relationship to adverse outcome are needed.
There are several limitations to this study. The study cohort is relatively small and non‐homogeneous, most patients studied were in NYHA II–III and only a minority were in class IV. Moreover, data that may affect patients' prognosis, such as changes during follow‐up in drugs or other treatments, was not collected, yet the patients were all well controlled and treated by a small number of HF specialists.
An additional controversial issue is the method we used for assessment of EPC levels. The CFU assay has been used by several authors, including in our previous studies.10,11,15,24,25,26 It is criticised by some authorities as it may be indicative of the proliferative capacity of EPC rather than their mere numbers. We have recently performed a comparative analysis of the currently used methods for EPC assessment in a cross‐sectional study of healthy volunteers and found that these methods are not correlated.27 We have therefore called for standardisation of the assay and, until this is done, we believe that the CFU method is a valid and reproducible method of assessing EPC levels, provided appropriate measures are taken to verify the identity of the colonies by immunohistochemical staining with relevant endothelial markers (fig 1).
In conclusion, we found that EPC levels are an independent predictor of mortality in patients with CHF. EPC levels are probably not influenced by the aetiology of HF, but rather correlate with the patient's clinical status reflected by the NYHA score. If these observations are supported by additional studies, EPC level determination could serve as an additional surrogate marker for the clinical follow‐up of patients with CHF.
Abbreviations
BNP - B‐type natriuretic peptide
CFU - colony‐forming unit
CHF - congestive heart failure
EPC - endothelial progenitor cell
HF - heart failure
hsCRP - high‐sensitivity C‐reactive protein
LVEF - left ventricular ejection fraction
NT‐pro‐BNP - N‐terminal pro‐B‐type natriuretic peptide
NYHA - New York Heart Association
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None declared.
References
- 1.McMurray J J, Pfeffer M A. The year in heart failure. J Am Coll Cardiol 2004442398–2405. [DOI] [PubMed] [Google Scholar]
- 2.Young J B. Management of chronic heart failure: what do recent clinical trials teach us? Rev Cardiovasc Med 20045(Suppl 1)S3–S9. [PubMed] [Google Scholar]
- 3.Fischer D, Rossa S, Landmesser U.et al Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 20052665–69. [DOI] [PubMed] [Google Scholar]
- 4.Kosiborod M, Smith G L, Radford M J.et al The prognostic importance of anemia in patients with heart failure. Am J Med 2003114112–119. [DOI] [PubMed] [Google Scholar]
- 5.Tsutamoto T, Wada A, Maeda K.et al Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 199796509–516. [DOI] [PubMed] [Google Scholar]
- 6.Yin W H, Chen J W, Jen H L.et al Independent prognostic value of elevated high‐sensitivity C‐reactive protein in chronic heart failure. Am Heart J 2004147931–938. [DOI] [PubMed] [Google Scholar]
- 7.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 20039702–712. [DOI] [PubMed] [Google Scholar]
- 8.Gill M, Dias S, Hattori K.et al Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res 200188167–174. [DOI] [PubMed] [Google Scholar]
- 9.Shintani S, Murohara T, Ikeda H.et al Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 20011032776–2779. [DOI] [PubMed] [Google Scholar]
- 10.George J, Goldstein E, Abashidze S.et al Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J 2004251003–1008. [DOI] [PubMed] [Google Scholar]
- 11.Hill J M, Zalos G, Halcox J P.et al Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003348593–600. [DOI] [PubMed] [Google Scholar]
- 12.Ghani U, Shuaib A, Salam A.et al Endothelial progenitor cells during cerebrovascular disease. Stroke 200536151–153. [DOI] [PubMed] [Google Scholar]
- 13.Vasa M, Fichtlscherer S, Aicher A.et al Number and migratory activity of circulating endothelial progenitor cells inversely correlates with risk factors for coronary artery disease. Circ Res 200189e1–e7. [DOI] [PubMed] [Google Scholar]
- 14.Rauscher F M, Goldschmidt‐Clermont P J, Davis B H.et al Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003108457–463. [DOI] [PubMed] [Google Scholar]
- 15.George J, Herz I, Goldstein E.et al Number and adhesive properties of circulating endothelial progenitor cells in patients with in‐stent restenosis. Arterioscler Thromb Vasc Biol 200323e57–e60. [DOI] [PubMed] [Google Scholar]
- 16.Valgimigli M, Rigolin G M, Fucili A.et al CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation 20041101209–1212. [DOI] [PubMed] [Google Scholar]
- 17.Bursi F, Weston S A, Redfield M M.et al Systolic and diastolic heart failure in the community. JAMA 20062962209–2216. [DOI] [PubMed] [Google Scholar]
- 18.Tepper O M, Galiano R D, Capla J M.et al Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 20021062781–2786. [DOI] [PubMed] [Google Scholar]
- 19.Orlic D, Kajstura J, Chimenti S.et al Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA 20019810344–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocher A A, Schuster M D, Szabolcs M J.et al Neovascularization of ischemic myocardium by human bone‐marrow‐derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 20017430–436. [DOI] [PubMed] [Google Scholar]
- 21.Jackson K A, Majka S M, Wang H.et al Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 20011071395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimmeler S, Aicher A, Vasa M.et al HMG‐CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3‐kinase/Akt pathway. J Clin Invest 2001108391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tepper O M, Capla J M, Galiano R D.et al Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow‐derived cells. Blood 20051051068–1077. [DOI] [PubMed] [Google Scholar]
- 24.Ghani U, Shuaib A, Salam A.et al Endothelial progenitor cells during cerebrovascular disease. Stroke 200536151–153. [DOI] [PubMed] [Google Scholar]
- 25.Thum T, Fraccarollo D, Galuppo P.et al Bone marrow molecular alterations after myocardial infarction: impact on endothelial progenitor cells. Cardiovasc Res 20067050–60. [DOI] [PubMed] [Google Scholar]
- 26.Sugawara J, Mitsui‐Saito M, Hayashi C.et al Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab 2005905329–5332. [DOI] [PubMed] [Google Scholar]
- 27.George J, Shmilovich H, Deutsch V.et al Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng 200612331–335. [DOI] [PubMed] [Google Scholar]