Abstract
Objectives
To quantify the prognostic utility of QRS and QTc interval prolongation in patients presenting with acute destabilised heart failure (ADHF) to the emergency department (ED).
Design
Prospective cohort study among patients enrolled in the B‐Type Natriuretic Peptide for Acute Shortness of Breath Evaluation (BASEL) study. QRS and QT intervals were measured in 173 consecutive patients with ADHF. QT interval was corrected using the Bazett formula. The primary end point was all‐cause mortality during the 720‐day follow‐up.
Results
QRS interval was prolonged (⩾120 ms) in 27% of patients, and QTc interval was prolonged (⩾440 ms) in 72% of patients. Baseline demographic and clinical characteristics were comparable in patients with normal and prolonged QRS or QTc intervals. A total of 78 patients died during follow‐up. Interestingly, the 720‐day mortality was similar in patients with prolonged and normal QTc (44% vs 42%, p = 0.546), but was significantly higher in patients with prolonged QRS interval than in those with normal QRS (59% vs 37%, p = 0.004). In Cox proportional hazards analysis, prolonged QRS interval was associated with a nearly twofold increase in mortality (HR 1.94, 95% CI 1.22 to 3.07; p = 0.005). This association persisted after adjustment for variables routinely available in the ED.
Conclusions
Prolonged QRS interval, but not prolonged QTc interval, is associated with increased long‐term mortality in patients with ADHF.
Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that electrocardiography be performed in all patients presenting with acute destabilised heart failure (ADHF).1,2,3 Electrocardiography is an important tool in the diagnosis of ADHF, particularly in the identification of the cause of acute decompensation including tachyarrhythmia and myocardial infarction.1,2,3,4 It is unknown whether electrocardiography also provides prognostic information in patients with ADHF. This would be attractive, as electrocardiography is routinely performed, easy, safe and inexpensive.
Recently, electrocardiography has received increasing recognition as a prediction tool in patients with chronic heart failure (HF). Prolongation of the QRS and the QTc interval have both been associated with increased mortality in chronic HF.5,6,7,8,9 QRS duration emerged as an important predictor of mortality in various cohorts of patients with chronic HF, including patients with systolic left ventricular dysfunction and patients with implantable cardioverter defibrillators (ICDs).5,6,7,8,9 Recently, prolonged QTc interval was suggested to be a powerful predictor of mortality in patients with advanced chronic HF.10 Kaplan–Meier survival rates were three times higher in patients with normal QTc interval than in those with prolonged QTc in a study excluding patients taking class III drugs for arrhythmia.10
It was the aim of this study to evaluate the prognostic utility of QRS and QTc interval prolongation in a contemporary cohort of consecutive patients presenting with ADHF to the emergency department (ED).
Methods
Setting and study population
This study specifically evaluated the prognostic utility of QRS and QTc interval prolongation in patients with ADHF enrolled in the B‐Type Natriuretic Peptide for Acute Shortness of Breath Evaluation (BASEL) study.11 The BASEL study was a prospective study conducted in the ED of the University Hospital in Basel, Switzerland. The study was carried out according to the principles of the Declaration of Helsinki and was approved by our local ethical committee. Written informed consent was obtained from all participants. A total of 452 patients were enrolled in the BASEL study; 217 patients were diagnosed as having ADHF according to current guidelines, and 173 patients (80%) qualified for this study.1,2,3,4 We excluded patients with pacemakers or ICDs and patients taking type III drugs for arrhythmia.10 The final discharge diagnosis of ADHF was based on clinical presentation and standard investigations, and adjudicated by an internal medicine specialist not involved in the ED care on the basis of all available medical records pertaining to the individual patient, including the response to treatment and autopsy data in those patients dying in hospital. B‐type natriuretic peptide (BNP) levels were measured and available for the final discharge diagnosis in 50% of patients.
QRS and QTc interval measurement
At the time of presentation to the ED, resting 12‐lead ECGs were recorded at a paper speed of 25 mm/s. A cardiologist blinded to clinical and survival data determined QRS and QT duration. QRS duration was measured using leads V3–V6.7
Prolonged QRS interval was prospectively defined as QRS interval ⩾120 ms.12 In accordance with the latest recommendations for clinical QT interval measurement, QT interval duration was recorded for three consecutive beats through leads II and V4.10,13 With calipers used on printed ECGs, each QT interval was measured from the beginning of the QRS complex to the visual return of the T wave to the isoelectric line. When the T wave was interrupted by the U wave, the end of the T wave was defined as the nadir between the T and the U waves. When the nadir was not clearly visible or the maximal T‐wave amplitude in leads II or V4 did not exceed 0.25 mV, the patient was excluded from the study. Heart rate correction was performed by the Bazett formula, and QTc interval duration was defined as the mean duration of all QTc intervals measured. Prolonged QTc interval was prospectively defined as QTc interval ⩾440 ms.10
End point and statistical analysis
All‐cause mortality was the primary end point of this study. Patients were contacted 6, 12 and 24 months after the initial presentation by telephone interview performed by a single trained researcher. In addition, referring doctors were contacted in the case of uncertainties regarding health status or hospitalisations. The administrative databases of the respective home towns were assessed to ascertain the vital status of those patients who could not be contacted by telephone. All information derived from contingent hospital readmission records or provided by the referring doctor or by the outpatient clinic was reviewed and entered into the computer database. The statistical analyses were performed using the SPSS V.13.0 software package. Comparisons were made using the t test, Mann—Whitney U test, Fisher's exact test and χ2 test as appropriate. All hypothesis testing was two tailed. The Kaplan–Meier method was used to analyse and compare survival in the prolonged and normal QRS and QTc groups. Cox proportional hazards analysis was used to identify predictors of death in univariate and multivariate analysis. Together with QRS and QTc prolongation, all baseline, demographic, clinical and laboratory variables routinely available in the ED were entered in a univariate Cox regression analysis. All variables associated with long‐term mortality in univariate analysis (p<0.05) were entered into the multivariate models.
Results
QRS interval was prolonged (⩾120 ms) in 27% of patients, and QTc interval was prolonged in 72% of patients. Baseline demographic and clinical characteristics were similar in patients with normal and prolonged QRS or QTc interval (table 1). Among patients with QRS prolongation, 58% had a left bundle branch block.
Table 1 Baseline patient characteristics.
| All patients (n = 173) | QRS <120 ms (n = 127) | QRS ⩾120 ms (n = 46) | p Value | QTc <440 ms (n = 47)* | QTc ⩾440 ms (n = 121)* | p Value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 75 (11) | 75 (11) | 76 (11) | 0.256 | 75 (12) | 75 (11) | 0.944 |
| Female sex | 78 (45) | 59 (47) | 19 (41) | 0.547 | 19 (40) | 56 (46) | 0.493 |
| History | |||||||
| Coronary artery disease | 120 (69) | 86 (68) | 34 (74) | 0.435 | 28 (60) | 89 (74) | 0.077 |
| Arterial hypertension | 111 (64) | 85 (67) | 26 (57) | 0.207 | 31 (66) | 79 (65) | 0.953 |
| COPD | 37 (21) | 27 (21) | 10 (22) | 0.946 | 11 (23) | 23 (19) | 0.524 |
| Diabetes mellitus | 60 (35) | 40 (32) | 20 (44) | 0.143 | 18 (38) | 41 (34) | 0.591 |
| Chronic kidney disease | 65 (38) | 47 (37) | 18 (39) | 0.799 | 17 (36) | 45 (38) | 0.824 |
| Symptoms | |||||||
| Paroxysmal nocturnal dyspnoea | 81 (47) | 56 (44) | 25 (54) | 0.232 | 21 (45) | 58 (48) | 0.705 |
| Nocturia | 73 (42) | 50 (39) | 23 (50) | 0.211 | 22 (47) | 51 (42) | 0.584 |
| Weight gain | 26 (15) | 20 (16) | 6 (13) | 0.660 | 9 (19) | 15 (12) | 0.262 |
| Vital status | |||||||
| Systolic blood pressure (mm Hg) | 149 (32) | 150 (33) | 146 (29 | 0.414 | 148 (26) | 151 (33) | 0.548 |
| Diastolic blood pressure (mm Hg) | 88 (22) | 90 (23) | 83 (20) | 0.041 | 89 (20) | 89 (23) | 0.971 |
| Heart rate (per minute) | 100 (26) | 102 (27) | 95 (22) | 0.150 | 105 (30) | 99 (24) | 0.149 |
| Temperature (°C) | 37.3 (0.9) | 37.3 (1.0) | 37.0 (0.8) | 0.047 | 37.5 (1.0) | 37.1 (0.9) | 0.029 |
| Signs | |||||||
| Tachypnoea (>20/min) | 79 (46) | 57 (45) | 22 (48) | 0.731 | 22 (47) | 53 (44) | 0.725 |
| Elevated JVP | 39 (23) | 25 (20) | 14 (30) | 0.135 | 11 (23) | 28 (23) | 0.971 |
| Hepatojugular reflux | 26 (15) | 17 (13) | 9 (20) | 0.315 | 10 (21) | 14 (12) | 0.107 |
| Rales | 107 (62) | 79 (62) | 28 (61) | 0.873 | 29 (62) | 74 (61) | 0.948 |
| Lower‐extremity oedema | 78 (45) | 56 (44) | 22 (48) | 0.663 | 24 (51) | 52 (43) | 0.344 |
| Laboratory tests | |||||||
| Ejection fraction (%)† | 41 (15) | 44 (15) | 33 (15) | 0.001 | 47 (16) | 39 (15) | 0.024 |
| Creatinine clearance (ml/min/1.73 m2) | 55 (29) | 56 (31) | 51 (22) | 0.277 | 58 (33) | 53 (28) | 0.376 |
| Haemoglobin (g/dl) | 12.9 (2.5) | 13.0 (2.5) | 12.7 (2.4) | 0.404 | 12.8 (2.5) | 13.0 (2.4) | 0.655 |
| Serum albumin (g/l) | 33 (6) | 33 (6) | 33 (6) | 0.504 | 33 (6) | 33 (6) | 0.475 |
| Troponin I (μg/l) | 0.6 (0.3–2.4) | 0.5 (0.3–2.1) | 1.0 (0.3–3.3) | 0.214 | 0.5 (0.3–1.9) | 0.6 (0.3–2.3) | 0.446 |
| BNP (pg/ml) | 805 (456) | 777 (452) | 877 (465) | 0.215 | 687 (469) | 833 (449) | 0.075 |
| Outcome | |||||||
| Hospitalisation | 153 (88) | 110 (87) | 43 (94) | 0.212 | 40 (85) | 108 (89) | 0.456 |
| Intensive care admission | 48 (28) | 36 (28) | 12 (26) | 0.769 | 13 (28) | 35 (29) | 0.870 |
| Time to discharge (days) | 13.4 (11.3) | 14.0 (12.2) | 12.0 (8.2) | 0.319 | 14.0 (13.3) | 12.6 (10.0) | 0.450 |
| 30‐day mortality | 22 (13) | 14 (11) | 8 (17) | 0.267 | 5 (11) | 16 (13) | 0.649 |
| 720‐day mortality | 37.5 (4.4) | 59.3 (7.3) | 0.004 | 41.8 (7.4) | 44.1 (4.5) | 0.546 |
BNP, B‐type natriuretic peptide; COPD, chronic obstructive pulmonary disease; JVP, jugular venous pressure.
Data are presented as mean (SD), median (interquartile range) or n (%) of patients. The p value is given for the comparison of patients with prolonged versus normal QRS and QTc intervals.
*QTc interval could not be determined in 5 (2.9%) patients.
†Determined in 117 patients during hospitalisation.
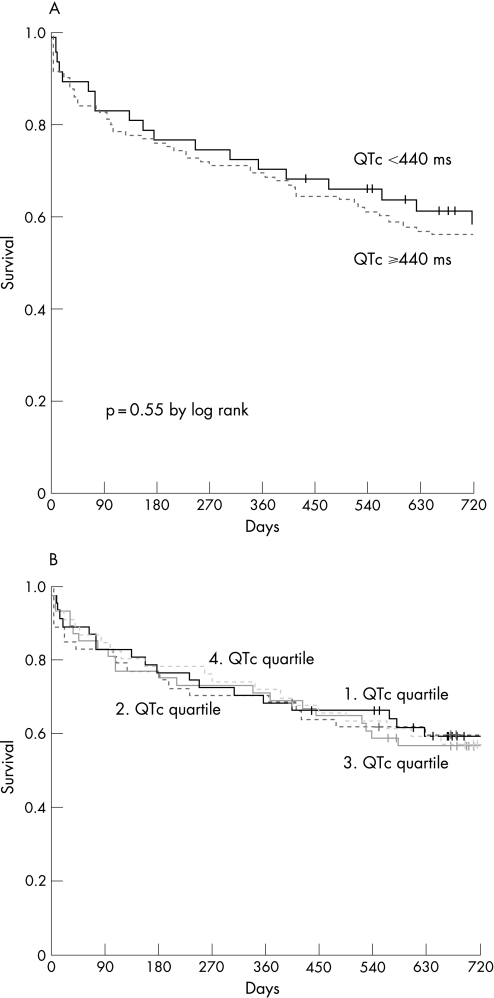
No patient was lost to follow‐up within the first 12 months. The median duration of follow‐up until the patient's death or last contact was 680 days. None of the patients had cardiac resynchronisation therapy initiated during the reported follow‐up period. A total of 78 patients died during follow‐up. Kaplan–Meier analysis revealed that the 720‐day mortality was comparable in patients with prolonged and normal QTc (44% vs 42%, p = 0.546; fig 1A). Mortality was also similar when patients were stratified according to QTc quartiles ((1) quartile QTc <411 ms; (2) quartile 411–436 ms; (3) quartile 436–462 ms; and (4) quartile >462 ms; fig 1B).
Figure 1 (A) Survival rates in patients with prolonged versus normal QTc interval. (B) Survival rates in relation to QTc quartiles (p = 0.995 by log rank).
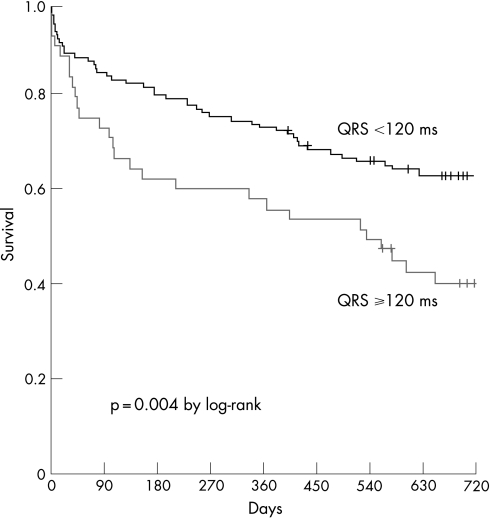
In contrast, the 720‐day mortality was significantly higher in patients with prolonged QRS interval than in those with normal QRS (59% vs 37%, p = 0.004; fig 2). In Cox proportional hazards analysis, prolonged QRS interval was associated with a nearly twofold increase in mortality (HR 1.94, 95% CI 1 22to 3.07; p = 0.005). This association persisted after multivariate adjustments in several models (table 2).
Figure 2 Survival rates in patients with prolonged vs normal QRS intervals.
Table 2 Univariate and multivariate analysis of prolonged QRS and QTc interval as predictors of all‐cause mortality.
| Univariate p | HR (95% CI) | Multivariate p (Model 1*) | HR (95% CI) | Multivariate p (Model 2†) | HR (95% CI) | Multivariate p (Model 3‡) | HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| QRS interval ⩾120 ms | 0.005 | 1.94 (1.22 to 3.07) | 0.016 | 1.77 (1.11 to 2.81) | 0.024 | 1.73 (1.07 to 2.78) | 0.058 | 1.67 (0.98 to 2.85) |
| QTc interval ⩾440 ms | 0.547 | 1.17 (0.70 to 1.97) |
HR, hazard ratio.
*Model 1: adjusting for age and coronary artery disease (CAD).
†Model 2: adjusting for age, history/comorbidity and vital signs. Variables in the final model included age, CAD, arterial hypertension, chronic obstructive pulmonary disease (COPD), systolic blood pressure and diastolic blood pressure.
‡Model 3 (echocardiography subgroup): adjusting for age, history/ comorbidity, vital signs and laboratory tests including left ventricular ejection fraction. Variables in the final model included age, CAD, arterial hypertension, COPD, systolic blood pressure, diastolic blood pressure, haemoglobin, albumin, creatinine clearance, B‐type natriuretic peptide and cardiac troponin I.
Echocardiography was performed in a subgroup of 117 patients (68%) during hospitalisation. QRS prolongation was present significantly more often in patients with significant left ventricular systolic dysfunction defined as a left ventricular ejection fraction (LVEF) of ⩽40% (38% vs 17%, p = 0.007). Also, BNP levels >500 pg/ml were present significantly more often in patients with LVEF of ⩽40% (72% vs 49%, p = 0.007).
LVEF was lower in both patients with prolonged QRS interval (33% vs 44% in patients with normal QRS, p = 0.001) and in patients with prolonged QTc interval (39% vs 47% in patients with normal QTc, p = 0.024).
When LVEF was added to the variables routinely available in the ED (echocardiography subgroup multivariate model 3) in these 117 patients, prolonged QRS interval fell short of being a significant predictor of mortality (p = 0.058). Only age and BNP remained independently associated with death in this subgroup.
Discussion
In patients presenting with ADHF to the ED, the ECG offers important diagnostic and prognostic information. In addition to providing clues regarding the cause of acute decompensation including tachyarrhythmia or myocardial infarction, QRS prolongation, but not QTc prolongation, identifies patients with a significantly increased risk of death during long‐term follow‐up. This finding has important clinical implications. First, patients with ADHF have a high mortality. Despite this, there are few data regarding long‐term outcome of patients with ADHF.1,2 Therefore, risk stratification in patients with ADHF is by far less well validated than in patients with chronic HF. Second, increased QRS duration can easily be detected on the 12‐lead ECG immediately on presentation. Third, rapid initiation of intensive care and several specific therapeutic interventions possibly including levosimendan14 or selective phosphodiesterase type 5 inhibition15 may particularly benefit high‐risk patients identified by prolongation of QRS duration. Fourth, in addition to a multidrug regimen and disease management programme for HF, biventricular pacing seems to provide symptomatic and prognostic benefit in HF patients with prolonged QRS interval.16 Given the dismal short‐ and long‐term prognosis of patients with prolonged QRS duration admitted for ADHF, this high‐risk subgroup of patients with ADHF should be evaluated for biventricular pacing. Until now, biventricular pacing has been appropriately validated in randomised controlled clinical trials only in patients with chronic HF.16 The high rates of mortality and hospital re‐admission in patients with ADHF provide a strong rationale to evaluate this treatment also in high‐risk ADHF.3
Prolonged QRS duration is due to delayed ventricular electrical activation. This changed electrical activation sequence may result in mechanical dyssynchrony.17,18 The resulting alteration in mechanical activation may lead to impaired haemodynamic performance and mitral regurgitation.18,19 The common consequence of these adverse effects is reduced ventricular pumping function and increased risk of heart failure and death. QRS prolongation could be interpreted as an easy and simple obtainable marker for significant left ventricular dysfunction. The association between QRS prolongation and impaired left ventricular systolic function might, at least in part, explain the association between QRS prolongation and mortality.
This study corroborates and extends previous studies on QRS duration prolongation as a predictor of mortality in patients with chronic HF.5,6,7,8,9 In a cohort of 100 patients referred for cardiac transplantation, 34% had QRS duration ⩾120 ms. This group did have more than twice the event rate (death or transplantation) as compared with the group with normal QRS interval.9 Similar results were obtained in 165 patients with ICD with HF. Although there was no difference in survival between patients with LVEF >35% and LVEF ⩽35%, mortality was significantly increased in patients with QRS duration ⩾150 ms.8 Our study included consecutive “real‐life patients admitted with ADHF. These patients were significantly older and have more extensive comorbidity than patients offered cardiac transplantation or ICD implantation. Therefore, it is reassuring to note that QRS duration does provide important prognostic information at both extremes of HF. In addition to HF, prolongation of QRS duration has been associated with increased risk of death in sinus node dysfunction18 and acute myocardial infarction.20
QT interval prolongation has been proposed as a risk factor for ventricular arrhythmia and death in an apparently healthy population,21 in patients after myocardial infarction22 and in patients with diabetes.23 Although a recent study in 241 selected patients with advanced chronic HF suggested that prolonged QTc interval was a powerful predictor of mortality,5 overall, the data on the predictive value of QTc interval duration in HF are scarce and variably negative and positive with differences in results only partly explained by differences in study details.24,25,26,27 Our study complements and extends these studies with regard to the use of QTc prolongation as a predictor of mortality in patients with chronic HF.5,24,25,26,27 Despite applying methods identical with those in the latest positive study in patients with chronic HF,10 we were unable to show any predictive value of QTc interval prolongation in patients with ADHF. This exemplifies the need for specific research targeting ADHF as a distinct clinical entity. Differences in age, comorbidity, non‐cardiac medication and heart rate may account for our negative result regarding QTc interval.
Potential limitations of this study merit consideration. First, owing to respiratory distress and tachypnoea, the quality of ECG recordings are often suboptimal in patients presenting with ADHF to the ED. This may have contributed to the negative result regarding QTc. Second, QRS and QT intervals were measured by a single cardiologist. Therefore, we cannot assess whether interobserver variability would affect the predictive value of QRS duration. As previous studies have reported interobserver and intraobserver correlations for QRS durations of 0.97 and 0.98, respectively, this issue seems to be minor. By using only lead II and V4 to determine QT interval length, we minimised the intra‐observer variability. Third, the onset of prolongation of QRS duration and potential disappearance during follow‐up was unknown in our cohort. Systematic ECG follow‐up might reveal whether patients have improved outcome once prolongation of QRS duration disappears. Fourth, LVEF was determined during hospitalisation in two‐thirds of patients. As a previous study of patients with chronic HF suggested that the prognostic information provided by QRS duration is additive to and independent of echocardiographic variables,7 further studies are necessary to evaluate whether QRS duration maintains its incremental prognostic value if echo data are available for all patients, and, particularly, whether echocardiography can be performed within the first hours of presentation to the ED.28 Unfortunately, this service is currently not available in most hospitals. Fifth, this was a post hoc analysis of a randomised controlled trial. However, as the BASEL study recruited consecutive patients, we are not aware of any bias potentially confounding our results.
Our analysis has three particular strengths. First, it included a large contemporary cohort of consecutive patients. Second, the study population was highly representative of the elderly population of patients with ADHF in clinical practice.4 Third, it was one of the first studies of patients with ADHF to provide long‐term follow‐up data.
In conclusion, prolonged QRS interval, but not prolonged QTc interval, is associated with increased long‐term mortality in patients with ADHF. Attention to this easily accessible parameter may improve patient management.
Abbreviations
ADHF - acute destabilised heart failure
BNP - B‐type natriuretic peptide
BASEL - B‐Type Natriuretic Peptide for Acute Shortness of Breath Evaluation
ED - emergency department
HF - heart failure
ICD - implantable cardioverter defibrillator
LVEF - left ventricular ejection fraction
Footnotes
Funding: This study was supported by research grants from the Swiss National Science Foundation and the Swiss Heart Foundation (to CM).
Competing interests: None.
References
- 1.Hunt S A, Baker D W, Chin M H.et al ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force On Practice Guidelines. Circulation 20011042996–3007. [DOI] [PubMed] [Google Scholar]
- 2.Task Force for the Diagnosis And Treatment Of Chronic Heart Failure, European Society of Cardiology: W J. Remme and K. Swedberg. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001221527–1560. [DOI] [PubMed] [Google Scholar]
- 3.The Task Force on Acute Heart Failure of the European Society of Cardiology Nieminen MS, Böhm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. Eur Heart J 200526384–416. [DOI] [PubMed] [Google Scholar]
- 4.Cleland J G, Swedberg K, Follath F.et al Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart failure survey programme—a survey on the quality of care among patients with heart failure in Europe.Part 1: patient characteristics and diagnosis. Eur Heart J 200324442–463. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson K D, Schwartz J S, Chen T M.et al Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997952660–2667. [DOI] [PubMed] [Google Scholar]
- 6.Xiao H B, Roy C, Fujimoto S.et al Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol 199653163–170. [DOI] [PubMed] [Google Scholar]
- 7.Bruch C, Gotzmann M, Stypmann J.et al Electrocardiography and Doppler echocardiography for risk stratification in patients with chronic heart failure: incremental prognostic value of QRS duration and a restrictive mitral filling pattern. J Am Coll Cardiol 2005451072–1075. [DOI] [PubMed] [Google Scholar]
- 8.Bode‐Schnurbus L, Bocker D, Block M.et al QRS duration: a simple marker for predicting cardiac mortality in ICD patients with heart failure. Heart 2003891157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freudenberger R, Sikora J A, Fisher M.et al Electrocardiogram and clinical characteristics of patients referred for cardiac transplantation: implications for pacing in heart failure. Clin Cardiol 200427151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrtovec B, Delgado R, Zewail A.et al Prolonged QTc interval and high B‐type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation 20031071764–1769. [DOI] [PubMed] [Google Scholar]
- 11.Mueller C, Scholer A, Laule Kilian K.et al Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004350647–654. [DOI] [PubMed] [Google Scholar]
- 12.Josephson M E.Clinical cardiac electrophysiology: techniques and interpretations. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2002
- 13.Toivonen L. More light on QT interval measurement. Heart 200287193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Follath F, Cleland J G, Just H.et al Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet 2002360196–202. [DOI] [PubMed] [Google Scholar]
- 15.Lepore J J, Maroo A, Bigatello L M.et al Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension. Chest 20051271647–1653. [DOI] [PubMed] [Google Scholar]
- 16.Bristow M R, Saxon L A, Boehmer J.et al The Comparison of Medical Therapy, pacing and Defibrillation in Heart Failure Investigators. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 20043502140–2150. [DOI] [PubMed] [Google Scholar]
- 17.Kass D A, Chen C H, Curry C.et al Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999991567–1573. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney M O, Hellkamp A S, Lee K L, Lamas G A, for the Mode Selection Trial (MOST) Investigators Association of prolonged QRS duration with death in a clinical trail of pacemaker therapy for sinus node dysfunction. Circulation 20051112418–2423. [DOI] [PubMed] [Google Scholar]
- 19.Bramlet D A, Morris K G, Coleman R E.et al Effect of rate‐dependent left bundle branch block on global and regional left ventricular function. Circulation 1983671059–1065. [DOI] [PubMed] [Google Scholar]
- 20.Bauer A, Watanabe M A, Barthel P.et al QRS duration and late mortality in unselected post‐infarction patients of the revascularization era. Eur Heart J 200627427–433. [DOI] [PubMed] [Google Scholar]
- 21.Schouten E G, Dekker J M, Meppelink P.et al QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation 1991841516–1523. [DOI] [PubMed] [Google Scholar]
- 22.Peters R W, Byington R P, Barker A.et al Prognostic value of prolonged ventricular repolarization following myocardial infarction: the BHAT experience: the BHAT Study Group. J Clin Epidemiol 199043167–172. [DOI] [PubMed] [Google Scholar]
- 23.Rossing P, Breum L, Major‐Pedersen A.et al Prolonged QTc interval predicts mortality in patients with type I diabetes mellitus. Diabet Med 200118199–205. [DOI] [PubMed] [Google Scholar]
- 24.Brendorp B, Elming H, Jun L.et al QTc interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with defetilide. Circulation 20011031422–1427. [DOI] [PubMed] [Google Scholar]
- 25.Spargias K S, Lindsay S J, Kawar G L.et al QT dispersion as apredictor of long‐term mortality in patients with acute myocardial infarction and clinical evidence of heart failure. Eur Heart J 1999201158–1165. [DOI] [PubMed] [Google Scholar]
- 26.Brooksy P, Batin P D, Nolan J.et al The relationship between QT intervals and mortality in ambulant patients with chronic heart failure: the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK‐HEART). Eur Heart J 1999201335–1341. [DOI] [PubMed] [Google Scholar]
- 27.Anastasiou‐Nana M I, Nanas J N, Karagounis L A.et al Relation of dispersion of QRS and QT in patients with advanced congestive heart failure to cardiac and sudden death mortality. Am J Cardiol 2000851212–1217. [DOI] [PubMed] [Google Scholar]
- 28.Lubien E, DeMaria A, Krishnaswamy P.et al Utility of B‐natriuretic peptide in detecting diastolic dysfunction. Comparison with Doppler velocity recordings. Circulation 2002105595–601. [DOI] [PubMed] [Google Scholar]