Abstract
Background
The association between B‐type natriuretic peptide (BNP) and coronary artery disease is not fully understood.
Objective
To assess whether ischaemia per se is a stimulus for BNP secretion.
Setting
University tertiary hospital, Spain (Virgen de la Arrixaca).
Design
Prospective interventional study.
Patients
11 patients (55 (9) years, left ventricular ejection fraction (LVEF) 45% (7%) with a non‐complicated anterior myocardial infarction (MI) and isolated stenosis of the left anterior descending (LAD) coronary artery, successfully treated by primary angioplasty.
Interventions
11.0 (0.9) days after MI, the LAD was occluded (20 min) for intracoronary infusion of progenitor cells. Blood samples were obtained from the femoral artery (peripheral circulation (PC)) and the coronary sinus (coronary circulation (CC)) immediately before and after coronary occlusion.
Main outcome measures
BNP (pg/ml) was measured and ischaemia biomarkers were monitored.
Results
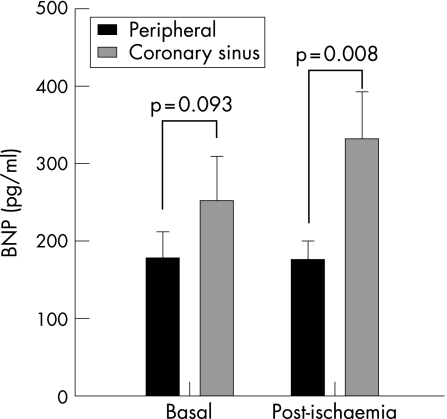
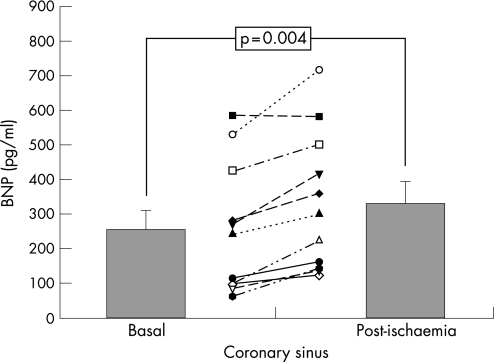
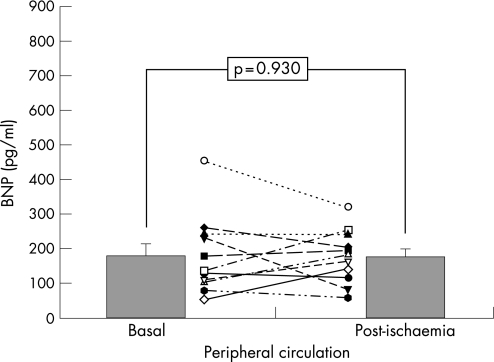
During coronary occlusion, all patients experienced transitory chest pain and ST‐segment dynamic changes. After coronary occlusion, lactic acid levels rose in CC (1.42 (0.63) –1.78 (0.68) ng/ml, p = 0.003). Myoglobin and cardiac troponin T did not differ in CC or PC at 24 h. No differences were found in LVEF (+0.18% (2.4)%, p = 0.86) and motion score index (–0.02 (0.06), p = 0.37). Before occlusion, BNP levels did not differ significantly in CC versus PC (253 (56) vs 179 (34), p = 0.093). After occlusion, BNP showed a significant increase in CC (vs 332 (61), p = 0.004), but no change occurred in PC (vs 177 (23), p = 0.93), and circulating BNP levels were higher in CC versus PC (p = 0.008).
Conclusions
In response to acute ischaemia, BNP levels immediately increase in coronary sinus but not at the peripheral level. BNP release in the coronary effluent may exert local beneficial effects.
B‐type natriuretic peptide (BNP) is a cardiac hormone secreted by cardiomyocytes in response to pressure and volume overload.1,2 Plasma BNP levels are used for diagnosis and monitoring of patients with heart failure, and BNP has emerged as a strong prognostic marker in patients with heart disease.3,4 After myocardial infarction, increases in BNP identifies patients at risk for left ventricular dysfunction and death.5,6 Recently, the prognostic role of BNP has been observed across the spectrum of acute coronary syndromes, including patients with non‐ST elevation myocardial infarction (MI) and unstable angina, as well as stable coronary artery disease*.7,8,9,10
The reason for the strong association between natriuretic peptides and mortality in CAD has so far not been fully understood. In this setting, high BNP levels may reflect (1) transient left ventricular dysfunction secondary to acute ischaemia, (2) the presence of several comorbidities such as hypertension or ventricular hypertrophy, or (3) the size or severity of the ischaemic insult, even when myocardial necrosis has not occurred. However, it remains unclear whether ischaemia‐induced BNP is due to ventricular dysfunction caused by ischaemia or due to myocardial ischaemia itself.
In this study, we assessed whether ischaemia per se is a stimulus for BNP secretion, and measured BNP levels at the coronary circulation (CC) and peripheral circulation (PC). We tested this hypothesis in a therapeutic human model of prolonged coronary occlusion.
Methods
Population and design
This is a substudy of a research trial of intracoronary autologous bone marrow transplantation.11 Patients with a first acute ST‐elevation MI Killip I, treated by primary angioplasty and coronary stenting of proximal left anterior descending (LAD) coronary artery within the first 12 h, were enrolled. In all cases, the location of MI was anterior and the only diseased coronary artery was the LAD. Patients were treated according to the current guidelines and all received aspirin, ACE inhibitors and β blockers.
Intracoronary administration of progenitor cells was performed between days 10 and 15 after MI. First, the left coronary artery and coronary sinus were catheterised. At this time, baseline blood samples were obtained from the femoral artery (PC) and the coronary sinus. Then, the balloon catheter was advanced into the previously implanted stent. The balloon was inflated at 2–4 atmospheres and the coronary blood flow was blocked during 10 periods of 2 min, for infusion of progenitor cell suspension. Within 5 min after the procedure, post‐ischaemia blood samples from the femoral artery and coronary sinus were obtained.
To confirm the presence of ischaemia, the occurrence of chest pain and ST‐segment dynamic changes (⩾1 mm) in at least two precordial leads during balloon occlusion were monitored, and blood samples for measurement of lactic acid were obtained from coronary circulation (CC). Myocardial necrosis was excluded by assessment of cardiac troponin T (cTnT) and myoglobin in the coronary sinus, and additionally every 6 h over the following 24 h in thePC.
Echocardiographic studies (Sonos 5500, Hewlett‐Packard, Palo Alto, California, USA) were carried out within 24 h before and after coronary occlusion. Standarised projections and measurements were made for the study of cardiac anatomy and ventricular function. Left ventricular ejection fraction (LVEF) was calculated by Simpson's method and wall motion score index was calculated as the mean score in a 16‐segment model (1, normal; 2, hypokinetic; 3, akinetic; 4, dyskinetic). This study was approved by the local ethics committee, and written informed consent was obtained from each patient.
Biochemical measurements
Blood samples for BNP measurement were collected in polystyrene tubes containing EDTA and aprotinin (500 KIU/ml), immediately placed on ice and centrifuged within 60 min. The plasma fraction was stored at−80°C until analysis. Plasma BNP concentrations were measured in duplicate with a specific solid‐phase sandwich immunoradiometric assay (Shionoria BNP Kit, CIS Bio International, Gifsur‐Yvette, France), which detects either BNP 1–32 or proBNP, as described previously.1 The detection limit was 2 pg/ml. Cross reactivity for atrial natriuretic peptide was specified by the manufacturer as <0.001%. The inter‐ and intra‐assay coefficient of variation was <6%. BNP values defined as normal by the manufacturer were <18.4 pg/ml. Blood samples for lactic acid measurement were collected in 125 μl plastic capillary tubes. Lactic acid levels were measured with selective electrodes on the ABL 735 blood gas analyser (Radiometer, Copenhagen, Denmark). Plasma levels of cTnT and myoglobin were measured by an electrochemiluminiscence immunoassay (Roche Diagnostics,, Mannheim, Germany). The lower detection limits were 0.01 and 21 ng/ml, respectively.
Statistical analysis
Data are expressed as mean values (SEM) for continuous variables and number (%) for categorical variables. BNP concentrations showed a skewed distribution, and given the small sample size (n = 11), we performed non‐parametric statistical analysis. The Wilcoxon rank sum test was used to compare changes after coronary occlusion in the evaluated parameters. The Mann–Whitney U test was used for two‐group unpaired comparisons. Correlations were performed using Pearson's correlation. A two‐sided probability value of p<0.05 was considered significant. Statistical analysis was performed using SPSS V.12.0 for windows.
Results
Eleven patients with a non‐complicated anterior MI and isolated stenosis of the LAD, successfully treated by primary angioplasty, were enrolled in the study. Table 1 shows the clinical and echocardiographic characteristics of the study population. The procedure of coronary occlusion was performed 11 (0.9) days after the infarction and was uneventful.
Table 1 Patients' characteristics.
| Age (years) | 54.5 (8.6) |
| Sex (male) (%) | 90 |
| Diabetes mellitus (%) | 40 |
| Hypertension (%) | 40 |
| Dyslipedemia (%) | 50 |
| Echocardiography | |
| LVEF (%) | 44.7 (7.0) |
| LVEDV (ml) | 116 (32) |
| LVEDD (mm) | 56 (12) |
| WMSI | 1.7 (0.2) |
| LA (mm) | 35 (4) |
LA, left atrium (diameter); LVEDD, left ventricle end‐diastolic diameter; LVEDV, left ventricle end‐diastolic volume; LVEF, left ventricular ejection fraction; WMSI, wall motion score index.
Data are expressed as mean (SD) unless otherwise specified.
During balloon inflation, all patients experienced transitory chest pain and ST‐segment dynamic changes in the ECG. In the coronary sinus, lactic acid levels increased from baseline to immediately after coronary occlusion (1.42 (0.63)–1.78 (0.68) ng/ml, p = 0.003), whereas no differences were detected in myoglobin levels (50.5 (11.3) vs 52.0 (13.3) ng/ml, p = 0.67) or in cTnT levels (0.34 (0.14) vs 0.35 (0.15) ng/ml, p = 0.31). The peak of necrosis markers measured in PC did not differ at 24 h from baseline. Myoglobin levels were 53.7 (15.7) and 48.9 (13.9) ng/ml (p = 0.27), and cTnT levels were 0.46 (0.21) and 0.44 (0.18) ng/ml (p = 0.31), respectively. As compared with baseline echocardiography, the post‐occlusion study did not show significant changes in LVEF (+0.18% (2.4%), p = 0.86) nor in wall motion score index (–0.02 (0.06), p = 0.37).
Baseline BNP levels were not significantly different in CC (253 (56) pg/ml) and in PC (179 (34) pg/ml; p = 0.093; fig 1). Post‐ischaemia BNP levels (immediately after transient coronary occlusion) significantly rose in CC (332 (61) pg/ml; p = 0.004 vs baseline; (fig 2), but no changes were found in PC (177 (23) pg/ml; p = 0.93 vs baseline; fig 3). Thus, after acute ischaemia, BNP levels were significantly higher in CC than in PC (177 (23) vs 332 (61), p = 0.008; fig 1). In the infused cell suspension, we detected BNP at low concentrations (range (8.4)–11.6 pg/ml), much lower than plasma levels. No significant correlations were observed between BNP levels and echocardiographic parameters (p>0.1), and only in the coronary sinus there was a significant correlation between post‐ischaemia BNP levels and the increase in lactic acid level (rs = 0.893, p<0.001).
Figure 1 Comparison between B‐type natriuretic peptide (BNP) concentrations in coronary sinus and peripheral circulation, before (basal) and immediately after the coronary occlusion (post‐ischaemia) (mean (SEM)).
Figure 2 Changes in B‐type natriuretic peptide (BNP) concentrations in coronary sinus, in response to coronary occlusion (mean (SEM)).
Figure 3 Changes in B‐type natriuretic peptide (BNP) concentrations in peripheral circulation, in response to coronary occlusion (mean (SEM)).
Discussion
The two main findings of this study are as follows: (1) after acute transient ischaemia, BNP is released in the CC and in this setting BNP levels do not correlate with ventricular dysfunction and (2) BNP levels immediately after ischaemia do not increase in the PC.
BNP has beneficial physiological properties, including balanced vasodilation, natriuresis and inhibition of both the sympathetic nervous system and the renin—angiotensin–aldosterone axis.12 These actions have been observed in patients with heart failure and it is well established that levels of these peptides are increased in ventricular volume or pressure overload.1,2 The triggering mechanism is believed to be an increase in wall stress, with accompanying myocyte stretch, that leads to BNP gene transcription.13 Nevertheless, even though the value of BNP is nowadays well characterised in heart failure and other heart diseases, the same does not apply to coronary disease and its ischaemic manifestations.
Our study shows for the first time at a clinical level the early release of BNP in CC after acute ischaemia. This local increase at a coronary level was previously observed in animal research models. In 1994, Toth et al14 demonstrated that hypoxic perfusion of isolated rat hearts led to a rapid increase of BNP immunoreactivity in coronary effluent, which provided the first evidence that stimuli other than increased wall stress might trigger BNP release.14 More recently, D'Souza et al15 also found that BNP concentrations in coronary effluent during reperfusion in isolated perfused rat hearts correlated with the duration of myocardial ischaemia and increased in a graded fashion with ischaemia severity. This study confirmed the rapid BNP release from ischaemic myocardium. The authors suggested that the increase of tissue BNP after 2 and 5 min of ischaemia probably reflects cleavage of the stored propeptide in response to ischaemia instead of mRNA transcription, which usually requires 1–2 h. In large animal models, Goetze et al16,17 demonstrated in swine that induction of myocardial ischaemia in hearts with normal ventricular function resulted in a rapid and significant rise in BNP gene expression in the affected tissues and in isolated perfused ventricular myocytes incubated in a hypoxic medium. As in previous experimental studies, we also found a correlation between lactate increase, such as ischaemia measure, and post‐ischaemia BNP levels.
This early BNP increase in CC after ischaemia may be interpreted within the multiple beneficial effects of BNP at the coronary level. BNP is a vasodilator in several vascular beds including coronary epicardial conductance arteries and coronary microvessels.18,19 Despite a decrease in coronary perfusion pressure, coronary artery blood flow increases, and coronary resistance and myocardial oxygen uptake decrease. Moreover, since BNP is highly expressed in coronary atherosclerotic lesions20 and has potent anti‐proliferative and anti‐migratory effects on vascular smooth muscle cells,21 it has a protective role in the pathogenesis of the coronary atherosclerotic disease. D'Souza et al15 showed that acute infusion of exogenous BNP is markedly protective against myocardial ischaemia reperfusion injury, leading to concentration‐dependent infarct size limitation. The mechanism of protection afforded by BNP is associated with increase in cGMP and seems to involve KATP channel opening. In this line of evidence, the early and selective BNP increase in the CC shown in our study supports a protective role of BNP against acute ischaemia.
At the level of PC, high BNP levels at rest are consistently associated with both a greater degree of inducible ischaemia in stress tests22,23,24,25,26,27,28 and a greater level of coronary disease in angiography.24,25,29 Furthermore, it has prognostic value in patients with both acute coronary syndromes8,30,31 and stable coronary artery disease.10,29 Studies assessing BNP response in models of stress‐induced ischaemia show discordances in their findings, partly explained by the differences in methods. Thus, several authors found that BNP change, measured immediately after echocardiographic dobutamine stress or 201Thallium single photon emission computed tomography, was not associated with the presence of inducible ischaemia.23,25 Others found that BNP increase was strongly associated with inducible ischaemia and correlated with its severity on nuclear imaging, as compared with healthy individuals.24,26,27,28 In our study, BNP levels in PC were not immediately modified after the coronary occlusion, although they were already increased at baseline, which might mask the changes that one might expect in BNP measured at a peripheral level after an acute ischaemic insult. In the study of Sabatine et al,27 the median of BNP increase was 14.2 pg/ml in patients with mild to moderate ischaemia and 23.7 pg/ml in those with severe ischaemia.
In a population undergoing coronary angioplasty with no previous infarction, Tateishi et al32found a significant increase of BNP in peripheral blood 24 h after the procedure (28 (26) –66 (65) pg/ml), as compared with a control group undergoing coronary angiography only. A plausible explanation for our findings and those of the aforementioned studies is a distinct physiology and kinetics in each circulation. Thus, ischaemia causes a direct and early BNP increase in CC, where it may exert a protective role against ischaemia, whereas the rise in peripheral blood would happen later as an equilibration process or be more variable and dependent on the severity of ischaemia and its consequences on ventricular function. The immediate stimulus for BNP release could be either ischaemia per se or local tissue stunning as a result of ischaemia. However, further studies are necessary to investigate the specific role of BNP in human CC.
The main limitations of this study were the small sample of patients and the lack of intraventricular pressure measurements during acute ischaemia. This measurement would have allowed us to rule out an increase in pressure as a cause of BNP secretion. However, the presence of selective secretion in the CC and the results of previous experimental studies make this a less important limitation. In vitro studies have shown that bone marrow endothelial cells are capable of producing BNP.33 We detected BNP in the cell infusion, in the normal range and much lower than plasma concentrations. Consequently, the observed BNP increase after coronary occlusion is not due to cellular infusion.
Conclusions
In conclusion, after transient myocardial ischaemia, BNP shows an immediate significant increase in CC not observed at the peripheral level. BNP release in the coronary effluent after acute ischaemia may exert local beneficial effects with possible therapeutic implications.
Acknowledgements
The study was supported in part by Grant‐2003 (PPC/01494/FS/03) from “Fundacion Seneca” from Consejeria de Economia, Industria e Innovacion de la Region de Murcia, Spain.
Abbreviations
BNP - B‐type natriuretic peptide
CC - coronary circulation
cTnT - cardiac troponin T
LAD - left anterior descending
LVEF - left ventricular ejection fraction
MI - myocardial infarction
PC - peripheral circulation
Footnotes
Competing interests: None.
References
- 1.Yasue H, Yoshimura M, Sumida H.et al Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation 199490195–203. [DOI] [PubMed] [Google Scholar]
- 2.Espiner E A. Physiology of natriuretic peptides. J Intern Med 1994235527–541. [DOI] [PubMed] [Google Scholar]
- 3.Koglin J, Pehlivanli S, Schwaiblmair M.et al Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol 2001381934–1941. [DOI] [PubMed] [Google Scholar]
- 4.Maisel A S, Krishnaswamy P, Nowak R M.et al Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002347161–167. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa N, Nakamura M, Aoki H.et al Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol 1996271656–1661. [DOI] [PubMed] [Google Scholar]
- 6.Omland T, Aakvaag A, Bonarjee V V.et al Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long‐term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N‐terminal proatrial natriuretic peptide. Circulation 1996931963–1969. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine M S, Morrow D A, de Lemos J A.et al Multimarker approach to risk stratification in non‐ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C‐reactive protein, and B‐type natriuretic peptide. Circulation 20021051760–1763. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos J A, Morrow D A, Bentley J H.et al The prognostic value of B‐type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 20013451014–1021. [DOI] [PubMed] [Google Scholar]
- 9.Bazzino O, Fuselli J J, Botto F.et al Relative value of N‐terminal probrain natriuretic peptide, TIMI risk score, ACC/AHA prognostic classification and other risk markers in patients with non‐ST‐elevation acute coronary syndromes. Eur Heart J 200425859–866. [DOI] [PubMed] [Google Scholar]
- 10.Omland T, Richards A M, Wergeland R.et al B‐type natriuretic peptide and long‐term survival in patients with stable coronary artery disease. Am J Cardiol 20059524–28. [DOI] [PubMed] [Google Scholar]
- 11.Aviles F F, San Roman J A, Garcia F J.et al Intracoronary stem cell transplantation in acute myocardial infarction. Rev Esp Cardiol 200457201–208. [PubMed] [Google Scholar]
- 12.Levin E R, Gardner D G, Samson W K. Natriuretic peptides. N Engl J Med 1998339321–328. [DOI] [PubMed] [Google Scholar]
- 13.Liang F, Gardner D G. Mechanical strain activates BNP gene transcription through a p38/NF‐kappaB‐dependent mechanism. J Clin Invest 19991041603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toth M, Vuorinen K H, Vuolteenaho O.et al Hypoxia stimulates release of ANP and BNP from perfused rat ventricular myocardium. Am J Physiol 1994266H1572–H1580. [DOI] [PubMed] [Google Scholar]
- 15.D'Souza S P, Yellon D M, Martin C.et al B‐type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol 2003284H1592–H1600. [DOI] [PubMed] [Google Scholar]
- 16.Goetze J P, Gore A, Moller C H.et al Acute myocardial hypoxia increases BNP gene expression. FASEB J 2004181928–1930. [DOI] [PubMed] [Google Scholar]
- 17.Goetze J P, Christoffersen C, Perko M.et al Increased cardiac BNP expression associated with myocardial ischemia. FASEB J 2003171105–1107. [DOI] [PubMed] [Google Scholar]
- 18.Brunner F, Wolkart G. Endothelial NO/cGMP system contributes to natriuretic peptide‐mediated coronary and peripheral vasodilation. Microvasc Res 200161102–110. [DOI] [PubMed] [Google Scholar]
- 19.Zellner C, Protter A A, Ko E.et al Coronary vasodilator effects of BNP: mechanisms of action in coronary conductance and resistance arteries. Am J Physiol 1999276H1049–H1057. [DOI] [PubMed] [Google Scholar]
- 20.Casco V H, Veinot J P, Kuroski de Bold M L.et al Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem 200250799–809. [DOI] [PubMed] [Google Scholar]
- 21.Schirger J A, Grantham J A, Kullo I J.et al Vascular actions of brain natriuretic peptide: modulation by atherosclerosis and neutral endopeptidase inhibition. J Am Coll Cardiol 200035796–801. [DOI] [PubMed] [Google Scholar]
- 22.Bibbins‐Domingo K, Ansari M, Schiller N B.et al B‐type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul Study. Circulation 20031082987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asada J, Tsuji H, Iwasaka T.et al Usefulness of plasma brain natriuretic peptide levels in predicting dobutamine‐induced myocardial ischemia. Am J Cardiol 200493702–704. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo B, Siepi D, Lupattelli G.et al Usefulness of brain natriuretic peptide levels to discriminate patients with stable angina pectoris without and with electrocardiographic myocardial ischemia and patients with healed myocardial infarction. Am J Cardiol 200494780–783. [DOI] [PubMed] [Google Scholar]
- 25.Weber M, Dill T, Arnold R.et al N‐terminal B‐type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J 2004148612–620. [DOI] [PubMed] [Google Scholar]
- 26.Foote R S, Pearlman J D, Siegel A H.et al Detection of exercise‐induced ischemia by changes in B‐type natriuretic peptides. J Am Coll Cardiol 2004441980–1987. [DOI] [PubMed] [Google Scholar]
- 27.Sabatine M S, Morrow D A, de Lemos J A.et al Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol 2004441988–1995. [DOI] [PubMed] [Google Scholar]
- 28.Rana B S, Davies J I, Band M M.et al B‐type natriuretic peptide can detect silent myocardial ischaemia in asymptomatic type 2 diabetes. Heart 200692916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kragelund C, Gronning B, Kober L.et al N‐terminal pro‐B‐type natriuretic peptide and long‐term mortality in stable coronary heart disease. N Engl J Med 2005352666–675. [DOI] [PubMed] [Google Scholar]
- 30.Bassan R, Potsch A, Maisel A.et al B‐type natriuretic peptide: a novel early blood marker of acute myocardial infarction in patients with chest pain and no ST‐segment elevation. Eur Heart J 200526234–240. [DOI] [PubMed] [Google Scholar]
- 31.Morrow D A, de Lemos J A, Sabatine M S.et al Evaluation of B‐type natriuretic peptide for risk assessment in unstable angina/non‐ST‐elevation myocardial infarction: B‐type natriuretic peptide and prognosis in TACTICS‐TIMI 18. J Am Coll Cardiol 2003411264–1272. [DOI] [PubMed] [Google Scholar]
- 32.Tateishi J, Masutani M, Ohyanagi M.et al Transient increase in plasma brain (B‐type) natriuretic peptide after percutaneous transluminal coronary angioplasty. Clin Cardiol 200023776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bordenave L, Georges A, Bareille R.et al Human bone marrow endothelial cells: a new identified source of B‐type natriuretic peptide. Peptides 200223935–940. [DOI] [PubMed] [Google Scholar]