Abstract
Background
Preoperative assessment of patients with aortic valve stenosis (AS) relies on the evaluation of AS severity (aortic valve area, AVA) and left ventricular ejection fraction (LVEF) by echocardiography, and of coronary artery anatomy by coronary angiography.
Aim
To evaluate the feasibility and accuracy of contrast‐enhanced multidetector computed tomography (MDCT), as a single non‐invasive preoperative test, for simultaneous evaluation of the AVA, LVEF and coronary status in patients with AS.
Methods
40 consecutive patients with AS scheduled for aortic valve replacement underwent transthoracic echocardiography, electrocardiogram (ECG)‐gated MDCT and coronary angiography within a time span of 1 week.
Results
MDCT measurements could be performed in all patients. A good correlation but a slight overestimation was observed between mean (SD) AVA measured by MDCT and by echocardiography (0.87 (0.22) vs 0.81 (0.20) cm2, p = 0.01; r = 0.77, p<0.001). Mean difference between methods was 0.06 (0.15) cm2. LVEF measured by MDCT correlated well with, and did not differ from, electrocardiographic measurements (59% (13%) vs 61% (10%), p = 0.34; r = 0.76, p<0.001; mean difference 1% (8%)). Coronary angiography displayed 33 lesions in 13 patients. MDCT correctly identified 26 of these 33 lesions and overestimated three <50% stenosis. On a segment‐by‐segment analysis, MDCT sensitivity, specificity, positive and negative predictive values were 79%, 99%, 90% and 98%, respectively. For each patient, MDCT had a sensitivity of 85% (11/13 patients), a specificity of 93% (25/27 patients) and positive and negative predictive values of 85% (11/13 patients) and 93% (25/27 patients), respectively.
Conclusion
MDCT can provide a simultaneous and accurate evaluation of the AVA, LVEF and coronary artery anatomy in patients with AS. In the near future, with technological improvements, MDCT could achieve an exhaustive and comprehensive preoperative assessment of patients with AS. In addition, for the assessment of AS severity in difficult cases, MDCT could be considered as an alternative to transoesophageal echocardiography or cardiac catheterisation.
Aortic stenosis (AS) is the most common valvular disease resulting in valve replacement.1 Preoperative assessment of patients with AS relies on the echocardiographic evaluation of AS severity (aortic valve area, AVA) and left ventricular function (left ventricular ejection fraction LVEF), and on the assessment of the coronary artery anatomy by coronary angiography.1,2
Cardiac multidetector computed tomography (MDCT) has shown promising results in the investigation of proximal coronary disease. The high negative predictive value of the technique seems ideally suited in the setting of preoperative patients with AS. It may help to rule out significant coronary artery disease (CAD),3,4 avoiding unnecessary coronary angiography in approximately half of the patients.5 However, reports on using MDCT in patients with AS are seldom available.6,7 Several reports also emphasised the importance of MDCT for the assessment of LVEF.8 Previous studies have shown that aortic valve morphology and the amount of aortic valve calcification can be assessed using MDCT,9,10,11 but few have focused on AVA and have shown promising results.12,13,14 Furthermore, whether these three parameters, AVA, LVEF and coronary anatomy, can be simultaneously obtained by MDCT has never been evaluated.
Thus, the aim of the present study was to assess the feasibility and accuracy of MDCT for the simultaneous evaluation of the AVA, LVEF and coronary artery status in patients with AS referred for aortic valve replacement.
Methods
Study population
Patients with AS referred to Bichat Hospital, Paris, France, for aortic valve replacement were potential candidates for the present study. Exclusion criteria were: (1) previous cardiac surgery; (2) could not evaluate the AVA by echocardiography; (3) presence of pacemaker; (4) severe respiratory impairment; (5) abnormal renal function (creatinine >2 mg/dl); and (6) allergy to iodine contrast media. All patients underwent transthoracic echocardiography, coronary angiography and electrocardiogram (ECG)‐gated MDCT within a time span of 1 week. Informed consent was obtained according to the standards of the joint committee for clinical investigation of Bichat Hospital.
MDCT
Scanning
MDCT scans were performed using a 16‐detector Philips scanner (Mx 8000 IDT 16) with commercially available cardiac reconstruction software. Contrast enhancement was achieved with 100–120 ml of iobitridol administered at a rate of 5 ml/s and flushed by saline at the same rate. ECG tracing was recorded during acquisition for retrospective reconstruction. Additional segmentation algorithm was used when the heart rate was >75 bpm, based on a segmented reconstruction of the images on two or four successive RR intervals. Image series were calculated every 12.5% of the RR interval from a phase centred around 0–50%, then at 75% and 87.5%. No β‐blockers were administered specifically for the purpose of MDCT examination. Radiation exposure was typically between 15 and 18 mSv. Temporal resolution was 0.210 ms and spatial resolution was 0.5×0.5×0.8 mm with a 0.4 mm overlap. Thirty‐two 0.75 mm thick slices were achieved per 360° rotation; table feed was automatically adjusted to the heart rate, resulting in a pitch of about 0.22.
Analysis
MDCT measurements were performed blinded to the results of other imaging modalities. Image quality was classified as good (optimal vascular enhancement, absence of breathing or ECG‐gated artefacts, clear coronary artery contours on at least one phase of the ECG), limited (one lacking criterion not precluding interpretation) or poor (one or more lacking criteria precluding interpretation).
Left ventricular end‐systolic and end‐diastolic volumes and LVEF: after the determination of end‐systolic phase (smaller left ventricular chamber as determined from a cine review in short axis) and end‐diastolic phase (larger left ventricular chamber as determined from a cine review in short axis), endocardial borders were manually traced in short axis. Left ventricular volumes were calculated using the Simpson's method applied to contiguous 5 mm thick multiplanar short‐axis reformations.
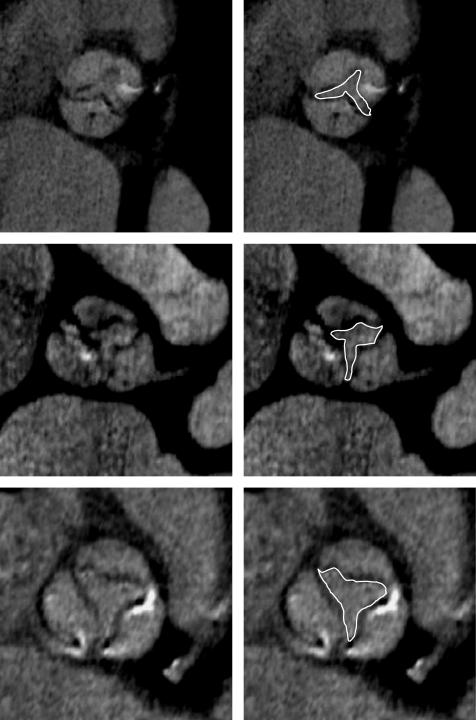
AVA: three different views (sagittal, coronal and transverse) were obtained from multiplanar reconstruction. AVA was measured by planimetry from a plane perpendicular to the axis of the aorta, at the tips of the leaflet at the time of maximal systolic opening (fig 1).
Coronary anatomy: cardiac phases were reviewed before coronary artery post‐processing. Presence and location of the stenosis was determined on a segment‐by‐segment basis relying on 10 segments.3 CAD was defined as ⩾50% stenosis or occlusion. If a coronary segment contained more than one lesion, the most severe lesion was recorded.
Figure 1 Examples of aortic valve area measured by multidetector computed tomography.
Echocardiographic assessment of AS severity was based on the AVA calculated using the continuity equation.2 Echocardiographic LVEF was evaluated on the basis of visual assessment and/or Simpson methods.15 Coronary angiograms were analysed by visual assessment. Results were expressed as normal, <50% stenosis or diseased (⩾50% stenosis or occlusion).
Statistical analysis
Quantitative variables were expressed as mean (SD). Comparisons of AVA and LVEF obtained by MDCT and echocardiography were analysed by using paired t‐tests, Pearson's correlation and Bland–Altman analysis.16 The diagnostic value of MDCT to assess for CAD was calculated on per‐segment and per‐patient basis and expressed as sensitivity, specificity and positive predictive values (PPVs) and negative predictive values (NPVs). A total of 20 patients were randomly selected from the study population to determine the reproducibility of MDCT measurements (AVA, LVEF and coronary anatomy assessment). Intraobserver and interobserver variability of AVA and LVEF measurements were calculated as mean (SD) difference. Concordance between the two observers for CAD evaluation was assessed by the κ value. Significance was defined at p<0.05.
Results
Population
In all, 50 consecutive patients were prospectively evaluated in this study. Out of 50, 10 patients were excluded because of abnormal renal function (n = 7) and the presence of a pacemaker (n = 3). The 40 remaining patients (25 men and 15 women, mean (SD) age 68 (11) years) were all in sinus rhythm (65 (9) bpm, range 49–90 bpm), although one displayed premature beats at the time of MDCT examination. Twelve patients were treated by β‐blockers before inclusion into the study. By echocardiography, AVA was 0.81 (0.20) cm2 (range 0.35–1.2 cm2) and LVEF 61% (10%) (range 30–82%). Twelve patients had an ejection fraction <60%. Four patients had a bicuspid aortic valve. Table 1 presents the clinical and echocardiographic characteristics of the study population.
Table 1 Clinical and echocardiographic characteristics.
| Age (years) | 68 (11) |
| Male | 25 (63%) |
| Symptoms | |
| Dyspnoea | 22 (55%) |
| Angina | 17 (43%) |
| Syncope and presyncope | 2 (5%) |
| LVEF (%) | 61 (10) |
| LV end‐diastolic diameter (mm) | 52 (8) |
| LV end‐systolic diameter (mm) | 34 (9) |
| Systolic pulmonary artery pressure, mm Hg | 38 (8) |
| Gradient, mm Hg | 51 (23) |
| AVA (cm2) | 0.81 (0.20) |
AVA, aortic valve area; LV, left ventricular; LVEF, left ventricular ejection fraction.
Data are presented as number of patients (%) or mean (SD).
MDCT
Examinations were judged as good in 32 and limited in 8 patients (3 because of heavily calcified aortic valves, 2 for insufficient distal right coronary artery assessment and 3 for distal circumflex assessment). There was no MDCT examination with poor image quality precluding analysis.
AVA
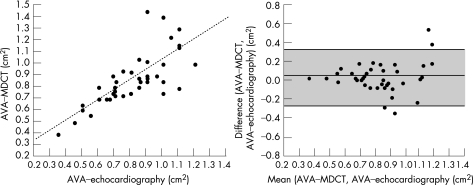
By MDCT, AVA mean (SD) was 0.87 (0.23) cm2 (range 0.40–1.45 cm2). A good correlation was observed with AVA measured by echocardiography (r = 0.77, p<0.001; fig 2A). Mean (SD) difference was 0.06 (0.15) cm2. Quality control plots showed a trend towards an overestimation of the AVA by MDCT (p = 0.02; fig 2B). Intraobserver and interobserver variability was 0.01 (0.12) and −0.04 (0.25) cm2, respectively.
Figure 2 Agreement between aortic valve area (AVA) measured by (A) multidetector row computed tomography and echocardiography as shown on a linear regression curve and (B) Bland–Altman analysis. The middle line represents the mean difference and the upper and lower lines ±2SD.
LVEF
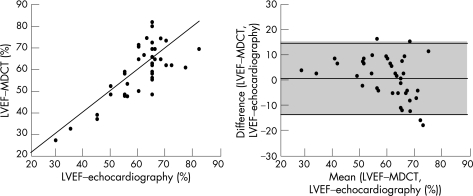
LVEF measured by MDCT was 59% (13%; range 26–83%), and correlated well with, and did not differ from, echocardiographic measurements (r = 0.76, p<0.001; p = 0.34; fig 3A). Mean (SD) difference was 1% (8%) and quality control plots showed no trend for over‐estimation or underestimation (fig 3B). Intraobserver and interobserver variability of MDCT measurements was low (–1 (4%) and –1% (5%), respectively).
Figure 3 Agreement between left ventricular ejection fraction (LVEF) assessed by (A) multidetector‐row computed tomography and echocardiography as shown on a linear regression curve and (B) Bland–Altman analysis. The middle line represents the mean difference and the upper and lower lines ± 2SD.
Coronary artery anatomy
Coronary angiography displayed 33 significant lesions in 13 patients: 24 stenosis >50% (11 left anterior descending coronary artery, 7 right coronary artery, 6 left circumflex coronary artery) and nine occlusions (3 left anterior descending coronary artery, 5 right coronary artery and 1 left circumflex coronary artery). MDCT correctly identified 26 of these 33 lesions and overestimated three non‐significant lesions in two patients (2 right coronary artery and one left anterior descending coronary artery stenosis, 2 of which contained a heavily calcified plaque).
On a segment‐by‐segment analysis, MDCT had a sensitivity of 79% (26/33) and a specificity of 99% (364/367). The PPV and NPV were 90% (26/29) and 98% (364/371), respectively. Per patient, MDCT had a sensitivity of 85% (11/13 patients), a specificity of 93% (25/27 patients), and PPV and NPV of 85% (11/13 patients) and 93% (25/27 patients), respectively.
The κ value was 0.86 indicating good interobserver agreement of MDCT assessment.
Discussion
This study shows that by using MDCT in patients with AS referred for surgery, simultaneous assessment of three important preoperative parameters (the valve area, the LVEF and the coronary artery anatomy) is feasible with an acceptable accuracy.
Assessment of AS severity usually relies on AVA calculated by echocardiography using the continuity equation.17 MDCT allows a three‐dimensional acquisition of the entire heart throughout the cardiac cycle and multiple plane reconstructions, which can be sliced in any plane as desired. It is thus possible to obtain a perfectly oriented parasternal short‐axis view of the AVA. Advances in technological features and retrospective ECG‐gating allows us to measure the AVA in mid‐late systole at the time of maximal opening. We have previously shown that this methodology provides accurate measurements of the mitral valve area18 and extend these findings to the AVA. We show that MDCT can provide acceptable measurements of AVA with good intraobserver and inter‐observer reproducibility. Our results compare favourably with other techniques, namely cardiac magnetic resonance imaging19,20 and transoesophageal echocardiography21,22 and three recently published reports using MDCT.12,13,14 It is worth noting that, similar to others, we have observed a slight overestimation of the AVA using MDCT compared with echocardiography. This overestimation may be related to the fact that echocardiography provides an assessment of the haemodynamic or effective orifice area and MDCT of the anatomical orifice area, which is generally somewhat larger.19 In addition, even if echocardiography and MDCT do not measure exactly the same parameter, our data scatter, similar to others,12,13,14 was significant (SD = 0.15 cm2). This result could be interpreted as a CT limitation but, in our view, convinces that no methods, including echocardiography, are highly precise. This underlines the need not only to consider AVA but also gradient and permeability index in the assessment of AS severity. In this regard, echocardiography remains indispensable.
Several studies have evaluated the accuracy of geometric assessment of the ventricular volumes from ECG‐gated MDCT data.8 We confirm and extend these findings to patients with AS and a wide range of LVEF. In this study, LVEF measured by MDCT did not differ from and correlated well with LEVF obtained by echocardiography. ECG‐gated MDCT allows several phases to be reconstructed retrospectively at the expense of no additional irradiation, and provides a true cine‐CT acquisition.
No significant CAD is observed in more than half of the patients with AS (67% in our study). In this study, in accordance to the literature, we observed a good negative predictive value of MDCT for the diagnosis of coronary lesions (93%). CAD could be ruled out using MDCT in 63% of our study population. Importantly, still two patients with significant CAD were missed using MDCT, which underlines the need for improving CT technology. On the other hand, the limited PPV of MDCT is often due to calcified lesions as illustrated in two cases in this study. This is an important limitation of CT angiography, and in those cases a coronary angiogram is mandatory. In contrast, valvular calcifications were not deleterious for AVA assessment. It is worth noting that the slight discrepancy between the per‐coronary segment and per‐patient analysis is related to the misdiagnosis of multiple lesions in the same patient.
Study limitations
Some limitations need do be underlined. First, only patients with severe AS were evaluated. Larger studies aiming at enrolling wider range of AS severity is desirable but this study focused on pre‐operative patients and simultaneous assessment of AVA, LVEF and coronary anatomy. Second, despite the fact that irregular rhythm was not an exclusion criterion, all our patients were in sinus rhythm. Tachycardia and/or irregular rhythm remains an important limitation of MDCT. Whether new 64‐slice CT and recently developed CT algorithms can overcome this limitation deserves further evaluation. Third, CT quality was judged as limited in 20% of our patients even if it did not preclude analysis. Fourth, with a 16‐detector CT system, there are still some limitations. CT assessment of coronary stenosis remains limited to proximal and middle main branches >2 mm.3 Volume coverage needs approximately a 20 s breath‐hold acquisition time that could be difficult to maintain. Radiation exposure remains high and a recent study emphasised reduction in dose parameters.23 Finally, the cumulative effects of iodinated contrast medium used for non‐conclusive MDCT and coronary angiography may be deleterious.
Clinical implications
In this study, we show that AVA assessment using MDCT is feasible and accurate. We are certainly not implying that MDCT can replace echocardiography for the assessment of AS severity, but to highlight that MDCT could be considered as an alternative to transoesophageal echocardiography (a semi‐invasive method) or to cardiac catheterisation (which presents some risks)24 performed for difficult but non‐rare cases (patients with poor echocardiographic windows, left ventricular outflow tract obstruction and so on). AVA can also be assessed in patients in whom MDCT is performed for other purposes such as patients with CAD.
In the preoperative field, MDCT confirms the degree of AS severity and provides an accurate assessment of LVEF. It also detects significant coronary artery lesions and, more importantly, can rule out significant coronary artery stenosis avoiding unnecessary coronary angiogram. It remains indicated in patients with inconclusive data or with a high suspicion of stenosis because of the average PPV. Therefore, in this population with a low or average CAD prevalence, MDCT may represent a useful preferred screening test before coronary angiography at the express condition that CT improves and reaches 100% NPV. In the near future, with technological improvements, MDCT could achieve a comprehensive and exhaustive preoperative assessment of patients with AS.
Conclusion
MDCT can provide a simultaneous and acceptable evaluation of AVA, LVEF and coronary artery anatomy in patients with AS. In the near future, with improvements in the quality of coronary artery imaging, MDCT may replace all preoperative tests. In addition, for the assessment of AS severity in difficult cases, MDCT could be considered as an alternative to transoesophageal echocardiography or cardiac catheterisation.
Abbreviations
AS - aortic stenosis
AVA - aortic valve area
CAD - coronary artery disease
LVEF - left ventricular ejection fraction
MDCT - multidetector‐row computed tomography
NPV - negative predictive value
PPV - positive predictive value
Footnotes
Competing interests: None.
References
- 1.Iung B, Baron G, Butchart E G.et al A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J 2003241231–1243. [DOI] [PubMed] [Google Scholar]
- 2.Bonow R, Carabello B, DeLeon A.et al ACC/AHA guidelines for the management of patients with valvular heart disease. Circulation 1998981949–1984. [DOI] [PubMed] [Google Scholar]
- 3.Nieman K, Cademartiri F, Lemos P.et al Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation 20021062051–2054. [DOI] [PubMed] [Google Scholar]
- 4.Ropers D, Baum U, Pohle K.et al Detection of coronary artery stenoses with thin‐slice multi‐detector row spiral computed tomography and multiplanar reconstruction. Circulation 2003107664–666. [DOI] [PubMed] [Google Scholar]
- 5.Peltier M, Trojette F, Sarano M E.et al Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three‐cuspid aortic valve. Am J Cardiol 20039197–99. [DOI] [PubMed] [Google Scholar]
- 6.Gilard M, Cornily J C, Pennec P Y.et al Accuracy of multislice computed tomography in the preoperative assessment of coronary disease in patients with aortic valve stenosis. J Am Coll Cardiol 2006472020–2024. [DOI] [PubMed] [Google Scholar]
- 7.Reant P, Brunot S, Lafitte S.et al Predictive value of noninvasive coronary angiography with multidetector computed tomography to detect significant coronary stenosis before valve surgery. Am J Cardiol 2006971506–1510. [DOI] [PubMed] [Google Scholar]
- 8.Juergens K U, Grude M, Fallenberg E M.et al Using ECG‐gated multidetector CT to evaluate global left ventricular myocardial function in patients with coronary artery disease. AJR Am J Roentgenol 20021791545–1550. [DOI] [PubMed] [Google Scholar]
- 9.Cowell S J, Newby D E, Burton J.et al Aortic valve calcification on computed tomography predicts the severity of aortic stenosis. Clin Radiol 200358712–716. [DOI] [PubMed] [Google Scholar]
- 10.Morgan‐Hughes G J, Owens P E, Roobottom C A.et al Three dimensional volume quantification of aortic valve calcification using multislice computed tomography. Heart 2003891191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willmann J K, Weishaupt D, Lachat M.et al Electrocardiographically gated multi‐detector row CT for assessment of valvular morphology and calcification in aortic stenosis. Radiology 2002225120–128. [DOI] [PubMed] [Google Scholar]
- 12.Alkadhi H, Wildermuth S, Plass A.et al Aortic stenosis: comparative evaluation of 16‐detector row CT and echocardiography. Radiology 200624047–55. [DOI] [PubMed] [Google Scholar]
- 13.Feuchtner G M, Dichtl W, Friedrich G J.et al Multislice computed tomography for detection of patients with aortic valve stenosis and quantification of severity. J Am Coll Cardiol 2006471410–1417. [DOI] [PubMed] [Google Scholar]
- 14.Bouvier E, Logeart D, Sablayrolles J L.et al Diagnosis of aortic valvular stenosis by multislice cardiac computed tomography. Eur Heart J 2006273033–3038. [DOI] [PubMed] [Google Scholar]
- 15.Lang R M, Bierig M, Devereux R B.et al Recommendations for chamber quantification. Eur J Echocardiogr 2006779–108. [DOI] [PubMed] [Google Scholar]
- 16.Altman D, Bland J. Measurement in medicine: the analysis of method comparison studies. Statistician 198332307 [Google Scholar]
- 17.Iung B, Gohlke‐Barwolf C, Tornos P.et al Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J 2002231252–1266. [DOI] [PubMed] [Google Scholar]
- 18.Messika‐Zeitoun D, Serfaty J M, Laissy J P.et al Assessment of the mitral valve area in patients with mitral stenosis by multislice computed tomography. J Am Coll Cardiol 200648411–413. [DOI] [PubMed] [Google Scholar]
- 19.Kupfahl C, Honold M, Meinhardt G.et al Evaluation of aortic stenosis by cardiovascular magnetic resonance imaging: comparison with established routine clinical techniques. Heart 200490893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John A S, Dill T, Brandt R R.et al Magnetic resonance to assess the aortic valve area in aortic stenosis: how does it compare to current diagnostic standards? J Am Coll Cardiol 200342519–526. [DOI] [PubMed] [Google Scholar]
- 21.Bernard Y, Meneveau N, Vuillemenot A.et al Planimetry of aortic valve area using multiplane transoesophageal echocardiography is not a reliable method for assessing severity of aortic stenosis. Heart 19977868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cormier B, Iung B, Porte J M.et al Value of multiplane transesophageal echocardiography in determining aortic valve area in aortic stenosis. Am J Cardiol 199677882–885. [DOI] [PubMed] [Google Scholar]
- 23.Hohl C, Muhlenbruch G, Wildberger J E.et al Estimation of radiation exposure in low‐dose multislice computed tomography of the heart and comparison with a calculation program. Eur Radiol 20061–6. [DOI] [PubMed]
- 24.Omran H, Schmidt H, Hackenbroch M.et al Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet 20033611241–1246. [DOI] [PubMed] [Google Scholar]