Abstract
Background
Tissue synchronisation imaging (TSI) is a new technique to assess left ventricular (LV) dyssynchrony.
Objectives
The value of using TSI to automatically assess LV dyssynchrony compared with manual assessment of LV dyssynchrony from colour‐coded tissue Doppler imaging (TDI), and to evaluate the value of TSI to predict response to cardiac resynchronisation therapy (CRT).
Methods
60 symptomatic patients with heart failure with depressed LV ejection fraction (LVEF) and QRS >120 ms were evaluated clinically and echocardiographically at baseline and after 6 months of CRT. LV dyssynchrony was measured manually using velocity tracings from the colour‐coded TDI and automatically using TSI. LV volumes and LVEF were assessed from two‐dimensional echocardiography. Clinical responders had to exhibit an improvement in New York Heart Association functional class by ⩾1 score and an improvement by ⩾25% in 6 min walking distance after 6 months. Reverse LV remodelling was defined as a reduction of ⩾15% LV end‐systolic volume.
Results
An excellent correlation was observed between LV dyssynchrony measured manually and automatically derived by TSI (r = 0.95, p<0.001). 34 patients showed clinical response after 6 months of CRT and 32 patients showed reverse remodelling. Baseline characteristics were comparable between responders and non‐responders, except for more extensive LV dyssynchrony in the responders: 78 (26) vs 29 (29) ms (p<0.001) as assessed manually, and 79 (29) vs 28 (27) ms (p<0.001) as assessed with TSI. Using a cut‐off value of 65 ms to define extensive LV dyssynchrony, TSI had a sensitivity of 81% with a specificity of 89% to predict reverse LV remodelling.
Conclusion
TSI allows automatic and reliable assessment of LV dyssynchrony and predicts reverse LV remodelling after CRT.
Cardiac resynchronisation therapy (CRT) is an attractive option in the treatment of patients with heart failure with poor left ventricular (LV) function and wide QRS complex, who remain symptomatic despite optimised medical treatment. Improvement in clinical end points (symptoms, exercise capacity, quality of life) and echocardiographic end points (eg, LV function and reverse remodelling) have been reported after CRT, with a reduction in the hospitalisation rate for decompensated heart failure, associated with an improvement in survival.1 Using the traditional selection criteria, a substantial percentage of patients do not respond to CRT. The assessment of LV dyssynchrony, as measured by echocardiographic techniques, has been proposed to improve identification of potential responders to CRT.2,3 Although not recognised as a gold standard, colour‐coded tissue Doppler imaging (TDI) has been used extensively for assessment of LV dyssynchrony.4,5,6,7,8,9 From the colour‐coded TDI images, myocardial velocity curves can be derived and the difference between peak systolic velocities in different regions has been shown to reflect LV dyssynchrony.4,5,6,7 These curves, however, are derived by manual post‐processing of the data and automated assessment may be preferred. Recently, tissue synchronisation imaging (TSI; GE Vingmed Ultrasound, Horton, Norway) has been introduced. TSI is a signal‐processing algorithm of tissue Doppler data to automatically detect peak positive velocities. The value of TSI to predict acute response to CRT was reported recently.10 Thus far, only one additional study reported the value of TSI to predict response to CRT.11
In this study, a head‐to‐head comparison between manual assessment of LV dyssynchrony (using colour‐coded TDI) and automated assessment of LV dyssynchrony (by TSI) was performed in 60 consecutive patients with heart failure. In addition, the value of TSI to predict response to CRT in these 60 patients was assessed.
Methods
Patients
The study population consisted of 60 patients with heart failure, scheduled for CRT, who where followed for at least 6 months. Inclusion criteria were severely symptomatic heart failure (New York Heart Association (NYHA) class III or IV) despite optimal medical treatment, depressed LVEF (<40%) and QRS width >120 ms (left bundle branch block or interventricular conduction delay) on the surface electrocardiogram. Patients with atrial fibrillation or a previously implanted pacemaker were excluded.
Assessment of functional status (at baseline and at 6 months follow‐up)
Patients were scored according to NYHA functional class by an experienced cardiologist. Subjects completed the Minnesota Living with Heart Failure Questionnaire, a 21‐question self‐administered instrument, with scores ranging from 0 to 5 for each question; higher scores indicate poorer quality of life.12 All patients performed a 6 min hall‐walk test to assess exercise capacity.13
Echocardiography
All patients underwent echocardiography before CRT implantation and again 6 months after. Studies were performed with commercially available echocardiographic equipment (VIVID 7, GE Vingmed Ultrasound, Horten, Norway). Global LV function was assessed by measuring LV end‐diastolic and end‐systolic volumes and LVEF, using the modified biplane Simpson's rule.
Colour‐coded TDI was performed using the apical four‐chamber view to assess longitudinal myocardial regional function. Gain settings, filters and pulse repetition frequency were adjusted to optimise colour saturation. Sector size and depth were optimised for the highest possible frame rate. At least two consecutive beats were recorded from each view and the images were digitally stored for offline analysis (EchoPac, GE Vingmed Ultrasound). The measurements were made by two independent observers blinded to the clinical outcome. In the basal septal and lateral segments, the time from the beginning of the QRS complex to peak systolic velocity (Ts) was measured; the delay in systolic velocities was considered to reflect LV dyssynchrony, as described previously.4,5
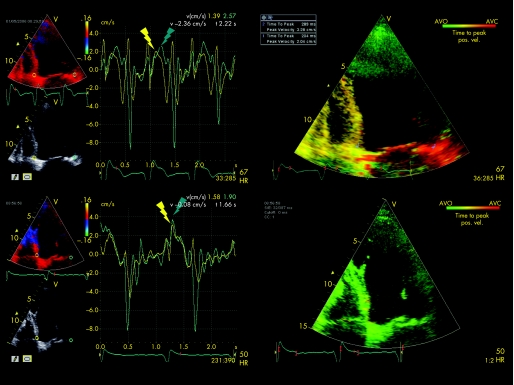
TSI is a parametric imaging tool derived from two‐dimensional tissue Doppler images. It automatically calculates Ts in every position in the image with reference to the QRS interval. The TSI algorithm detects positive velocity peaks within a specified time interval, and the colour coding ranges from green (earliest) to red (latest) within this interval. Using the user‐defined event‐timing tool, time from onset of the QRS complex to the aortic valve opening and closure was first measured in a separately recorded pulsed Doppler spectrum. To prevent the TSI system of measuring peak systolic velocities outside the ejection phase, the event‐timing tool was used to manually adjust start and end times of the TSI. The start time was set at aortic valve opening and the end time at aortic valve closure. The automatic Ts detection, which is the basis for TSI, was performed within this time period. A quantitative measurement tool allows calculation of the median Ts within a 6 mm sample volume manually positioned within the two‐dimensional TSI image. The sample volume was placed at the basal septal and lateral wall and LV dyssynchrony was calculated automatically by the TSI software. Figure 1 shows an example of echocardiographic assessment of LV dyssynchrony.
Figure 1 Example of a patient with ischaemic cardiomyopathy who underwent cardiac resynchronisation therapy implantation. The upper left panel shows the pre‐implantation colour‐coded velocity curves derived from the basal septal (yellow) and lateral (green) segments. There is a delay of 90 ms between the septal and lateral peak systolic velocities indicating left ventricular dyssynchrony. The upper right panel shows the tissue synchronisation imaging (TSI) pre‐implantation analysis. The red colour at the basal lateral wall indicates (90) ms delay in mechanical activation. The automatically calculated difference in peak systolic velocities between the septal and lateral wall is 85 ms. The lower left panel shows the post‐implantation velocity curves of the basal septum and lateral wall. No delay remains between the peak systolic velocities of the septum and lateral wall. The lower right panel shows the TSI analysis after implantation. The absence of a red‐coloured region indicates absence of late activation. pos vel, positive velocity.
Implantation of CRT device
The LV pacing lead was inserted transvenously through the subclavian route. First, a coronary sinus venogram was obtained during occlusion of the coronary sinus with a balloon catheter. Next, the LV pacing lead was inserted into the coronary sinus with the help of an 8F guiding catheter and positioned as far as possible in the venous system, preferably in the posterolateral vein. The right atrial and ventricular leads were positioned conventionally. When a conventional indication for an internal defibrillator existed, a CRT‐D device was implanted. At implantation, both the sensing and pacing thresholds (at pulse duration of 0.5 ms) of the LV pacing lead were measured. For each patient, the AV interval was adjusted to maximise the mitral inflow duration with pulsed‐wave Doppler echocardiography. No adjustments were made to the V–V interval during the first 6 months of CRT. The final position of the LV pacing lead was assessed with cine fluoroscopy.
Definition of clinical responders and reverse LV remodelling
Patients were subsequently divided into clinical responders and non‐responders, on the basis of an improvement in NYHA functional class by ⩾1 score and an improvement by ⩾25% in 6 min walking distance. Reverse LV remodelling was defined as a reduction of ⩾15% LV end‐systolic volume (echocardiographic responders).
Statistical analysis
All analyses were performed with the statistical software program SPSS V.12.0.1. Continuous data are presented as mean (SD). Categorical data are presented as absolute number or percentages. Comparisons between responders and non‐responders were carried out using the independent‐samples t test; comparisons between pre‐ and post‐implantation characteristics were carried out using the paired‐samples t test. Correlations between variables were studied using Pearson's correlations. Bland–Altman analysis was used to compare the manual (using TDI) and automatic (using TSI) assessments of LV dyssynchrony.14 Sensitivity and specificity were calculated to predict clinical response and LV reverse remodelling after CRT using a LV dyssynchrony cut‐off value of 65 ms.5 The significance level was set at p = 0.05.
Results
Study population
A total of 60 patients were included (mean (SD) age 68 (8) years, 80% men). All patients had severe LV dysfunction (LVEF 23 (7%). Aetiology of heart failure was ischaemic in 46 (77%) patients and non‐ischaemic in 14 (23%) patients. Treatment consisted of ACE inhibitors and/or angiotensin II receptor antagonists (87%), diuretics (92%), spironolactone (37%), β blockers (57%) and digoxin (27%). Three patients died of heart failure before the 6‐month follow‐up and were considered as non‐responders.
Pacemaker implantation
CRT device and lead implantation was successful in all patients without major complications (Contak TR or CD, Guidant, and Insync III or CD, Medtronic). Two types of LV leads were used: Easytrack 4512‐80 (Guidant) or Attain‐SD 4189 (Medtronic).
Improvement after CRT
Before implantation, 52 patients were in NYHA class III and 8 in NYHA class IV. After implantation, 6 patients were in NYHA class I, 37 in class II, 15 in class III and 2 in class IV. The mean (SD) NYHA class decreased from 3.1 (0.3) to 2.2 (0.7; p<0.001). Minnesota score decreased from 35 (15) to 22 (16; p<0.001) and 6 min walking distance increased from 305 (101) to 390 (126) m (p<0.001). LVEF improved from 24% (8%) to 29% (9%; p<0.001), with a reduction in LV end‐diastolic volume from 245 (89) to 220 (90) ml (p<0.001) and LV end‐systolic volume from 190 (83) to 161 (80) ml (p<0.001).
Accuracy of TSI to measure LV dyssynchrony
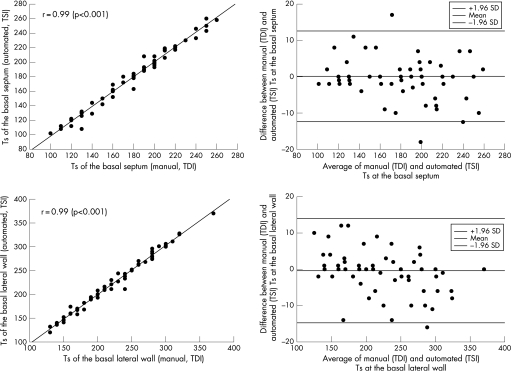
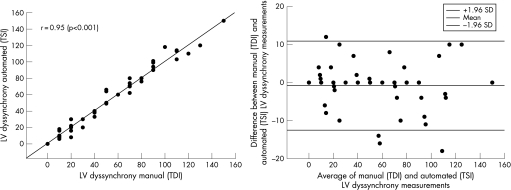
An excellent correlation existed between Ts measured by manual analysis of myocardial velocity curves (from colour‐coded TDI) and Ts measured automatically by TSI (fig 2). Also, a strong correlation was observed between manually assessed LV dyssynchrony (from colour‐coded TDI) and automatically assessed LV dyssynchrony (assessed by TSI): r = 0.95, p<0.001. Figure 3 also depicts a Bland–Altman plot comparing LV dyssynchrony measured manually from colour‐coded TDI and automatically assessed using TSI. Limits of agreement were −13 and +11 ms.
Figure 2 Scatter plot (upper left panel) and Bland–Altman plot (upper right panel) comparing manual (TDI) and automated (TSI) measurements of time‐to‐peak systolic velocities (Ts) at the basal septum. Scatter plot (lower left panel) and Bland–Altman plot (lower right panel) comparing manual (TDI) and automated (TSI) measurements of time‐to‐peak systolic velocities at the basal lateral wall. Pearson's correlations are reported in the left upper corner of the scatter plots.
Figure 3 Scatter plot (left panel) and Bland–Altman plot (right panel) comparing left ventricular (LV) dyssynchrony measured manually (tissue Doppler imaging (TDI)) and automatically (tissue synchronisation imaging (TSI)).
Clinical response
A total of 34 (57%) patients were considered to be clinical responders. At baseline, the only significant difference between clinical responders and non‐responders was LV dyssynchrony, measured either manually (using colour‐coded TDI) or automatically (using TSI, table 1). In clinical responders (table 2), mean NYHA functional class improved from 3.1 (0.3) to 1.9 (0.4), whereas it remained unchanged in non‐responders. The 6 min walking distance improved from 287 (96) to 441 (96) m in clinical responders and remained unchanged in non‐responders.
Table 1 Baseline characteristics of clinical responders versus non‐responders.
| Responders (n = 34) | Non‐responders (n = 26) | p Value | |
|---|---|---|---|
| Age (years) | 67 (10) | 68 (9) | NS |
| Gender (M/F) | 27/7 | 21/5 | NS |
| Ischaemic/idiopathic | 24/10 | 22/4 | NS |
| NYHA III/IV | 31/3 | 21/5 | NS |
| 6 min walking (m) | 287 (96) | 315 (116) | NS |
| QoL score | 36 (15) | 37 (17) | NS |
| LVEF (%) | 23 (7) | 24 (8) | NS |
| LVEDV (ml) | 266 (93) | 219 (74) | 0.04 |
| LVESV (ml) | 207 (85) | 170 (74) | NS |
| LV dyssynchrony assessed manually from colour‐coded TDI (ms) | 78 (26) | 29 (29) | <0.001 |
| LV dyssynchrony assessed automatically from TSI (ms) | 79 (29) | 28 (27) | <0.001 |
F, female; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; M, male; NYHA, New York Heart Association; QoL, quality of life; TDI, tissue Doppler imaging; TSI, tissue synchronisation imaging.
Data are presented as mean (SD).
Table 2 Clinical responders (n = 34) versus non‐responders (n = 26), clinical and echocardiographic variables before and 6 months after cardiac resynchronisation therapy implantation.
| Responders | Non‐responders | p Value | |
|---|---|---|---|
| NYHA class | |||
| Baseline | 3.1 (0.3) | 3.2 (0.4) | NS |
| Follow‐up | 1.9 (0.4)* | 2.7 (0.7) | <0.001 |
| 6 min walking (m) | |||
| Baseline | 287 (96) | 315 (116) | NS |
| Follow‐up | 441 (96)* | 316 (130) | <0.001 |
| QoL score | |||
| Baseline | 36 (14) | 37 (17) | NS |
| Follow‐up | 14 (10)* | 33 (17) | <0.001 |
| LVEF (%) | |||
| Baseline | 23 (7) | 24 (8) | NS |
| Follow‐up | 32 (9)* | 25 (9) | 0.01 |
| LVEDV (ml) | |||
| Baseline | 266 (93) | 219 (74) | NS |
| Follow‐up | 224 (93)* | 215 (88) | NS |
| LVESV (ml) | |||
| Baseline | 207 (85) | 170 (74) | NS |
| Follow‐up | 156 (77)* | 167 (85) | NS |
LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; QoL, quality of life; TDI, tissue Doppler imaging; TSI, tissue synchronisation imaging.
Data are presented as mean (SD) or number (%).
*p<0.001 baseline vs follow‐up.
Echocardiographic response
Besides a significant improvement in clinical parameters after CRT, the majority of the clinical responders (85%) also exhibited reverse remodelling as indicated by the reduction of LV end‐diastolic and end‐systolic diameters (table 2). In clinical responders, the LV dyssynchrony had decreased from 76 (29) to 26 (22) ms (p<0.001), whereas in non‐responders, LV dyssynchrony remained unchanged: 28 (27) vs 31 (28) ms (p = NS). At baseline, the only difference between patients showing reverse LV remodelling (n = 32) and patients without reverse remodelling (n = 28) was LV dyssynchrony, measured either manually (75 (26) vs 37 (36) ms, p<0.05) or using TSI (78 (26) vs 37 (35) ms, p<0.001).
Prediction of clinical response and reverse remodelling
In recent publications, the optimal cut‐off value for (manually measured) LV dyssynchrony to predict clinical improvement and reverse remodelling was 65 ms.5,15 When the same cut‐off value was applied to the TSI data, a sensitivity of 80% with a specificity of 92% were obtained to predict clinical response. In addition, a cut‐off value of 65 ms enabled prediction of reverse LV remodelling with a sensitivity of 81% and a specificity of 89%.
Discussion
The main findings of this study are twofold. First, using TSI, LV dyssynchrony can be reliably assessed in CRT candidates. Second, using TSI, a cut‐off value of 65 ms for LV dyssynchrony was highly predictive for clinical response and reverse remodelling after CRT.
Various studies have shown that LV dyssynchrony is an important determinant of response to CRT, and different techniques to detect and quantify LV dyssynchrony are currently under investigation.3,4,5,6,7,8,9,15 Until recently, measurement of LV dyssynchrony required manual post‐processing of the colour‐coded TDI images, and automated assessment may be preferred. The TSI algorithm automatically detects positive velocity peaks within a specified time interval. Some authors used the first half of the ejection phase,16 others used the interval between aortic valve opening and closure or even extend the interval into diastole to detect post‐systolic shortening.11 In this study, we used the interval between aortic valve opening and closure as suggested by Yu et al.11 The extent of LV dyssynchrony is generated automatically, both semiquantitatively (with colour coding, with red‐coloured areas indicating late activation) and quantitatively (by placing the sample volume at the desired LV segment).
Few studies have reported on the value of TSI for the automatic analysis of LV dyssynchrony. Gorcsan et al10 demonstrated, in 29 patients who underwent CRT, that differences in baseline time‐to‐peak velocities of opposing ventricular walls by TSI were greater in patients with an acute haemodynamic improvement. Yu et al11 evaluated 56 heart failure patients at baseline and 3 months after CRT. The authors used TSI to automatically assess Ts in the 12 different myocardial segments, and demonstrated an excellent correlation between manually (using colour‐coded TDI) and automatically (using TSI) measured Ts for the different segments; in this study, excellent agreement, for the large majority of patients, between manually and automatically assessed Ts was confirmed (fig 2). Moreover, Yu et al11 demonstrated that LV dyssynchrony derived from the 12 segments was highly predictive for LV reverse remodelling at follow‐up.
Similar results were obtained in this study; patients with LV dyssynchrony on TSI showed an improvement in NYHA class, 6 min walking distance and quality‐of‐life score at 6 months after CRT implantation. In addition, an improvement in LVEF with a reduction in LV volumes was observed at the 6‐month follow‐up. A cut‐off value for substantial LV dyssynchrony of 65 ms was used; this cut‐off value was derived from earlier studies using colour‐coded TDI.5,11 Application of that cut‐off value resulted in a sensitivity and specificity of 80% to predict response to CRT.5 The results in this study indicate that similar results can be obtained when the 65 ms cut‐off value is applied to TSI data; using this cut‐off value a sensitivity and a specificity of 80% and 92%, respectively, to predict clinical improvement were obtained. Moreover, comparable sensitivity and specificity to predict reverse LV remodelling were obtained.
Conclusion
Automated assessment of LV dyssynchrony with TSI is reliable, with excellent correlation with manually assessed LV dyssynchrony using colour‐coded TDI. LV dyssynchrony ⩾65 ms yields a high sensitivity and specificity to predict clinical response and reverse LV remodelling after 6 months of CRT.
Acknowledgements
GBB is supported by the Dutch Heart Foundation grant 2002B109.
Abbreviations
CRT - cardiac resynchronisation therapy
LV - left ventricular
LVEF - left ventricular ejection fraction
NYHA - New York Heart Association
TDI - tissue Doppler imaging
TSI - tissue synchronisation imaging
Ts - systolic velocity
Footnotes
Competing interests: None declared.
References
- 1.Freemantle N, Tharmanathan P, Calvert M J.et al Cardiac resynchronisation for patients with heart failure due to left ventricular systolic dysfunction—a systematic review and meta‐analysis. Eur J Heart Fail 20068433–440. [DOI] [PubMed] [Google Scholar]
- 2.Bax J J, Abraham T, Barold S S.et al Cardiac resynchronization therapy: part 1—issues before device implantation. J Am Coll Cardiol 2005462153–2167. [DOI] [PubMed] [Google Scholar]
- 3.Bank A J, Kelly A S. Tissue Doppler imaging and left ventricular dyssynchrony in heart failure. J Card Fail 200612154–162. [DOI] [PubMed] [Google Scholar]
- 4.Bax J, Marwick T H, Molhoek S G.et al Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end‐stage heart failure before pacemaker implantation. Am J Cardiol 2003921238–1240. [DOI] [PubMed] [Google Scholar]
- 5.Bax J J, Bleeker G B, Marwick T H.et al Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004441834–1840. [DOI] [PubMed] [Google Scholar]
- 6.Yu C M, Chau E, Sanderson J E.et al Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002105438–445. [DOI] [PubMed] [Google Scholar]
- 7.Yu C M, Fung W H, Lin H.et al Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 200291684–688. [DOI] [PubMed] [Google Scholar]
- 8.Bader H, Garrigue S, Lafitte S.et al Intra‐left ventricular electromechanical asynchrony; a new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 200443248–256. [DOI] [PubMed] [Google Scholar]
- 9.Sogaard P, Egeblad H, Kim W Y.et al Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long‐term cardiac resynchronization therapy. J Am Coll Cardiol 200240723–730. [DOI] [PubMed] [Google Scholar]
- 10.Gorscan J I I I, Kanzaki H, Bazaz R.et al Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol 2004931178–1181. [DOI] [PubMed] [Google Scholar]
- 11.Yu C M, Zhang Q, Wing‐Hong Fung J.et al A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol 200545677–684. [DOI] [PubMed] [Google Scholar]
- 12.Rector T S, Cohn J N. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 19921241017–1025. [DOI] [PubMed] [Google Scholar]
- 13.Refsgaard J. This is a walking test not a talking test: the six minute walking test in congestive heart failure. Eur Heart J 200526749–750. [DOI] [PubMed] [Google Scholar]
- 14.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986327307–310. [PubMed] [Google Scholar]
- 15.Bleeker G B, Kaandorp T A, Lamb H J.et al Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006113969–976. [DOI] [PubMed] [Google Scholar]
- 16.Murphy R T, Sigurdsson G, Mullamalla S.et al Tissue synchronization imaging and optimal left ventricular pacing site in cardiac resynchronization therapy. Am J Cardiol 2006971615–1621. [DOI] [PubMed] [Google Scholar]