Abstract
Objectives
To evaluate the risk of cardiovascular disease in individuals with coeliac disease (CD).
Design
Swedish national hospital‐based register data were used to identify 13 358 individuals who had been diagnosed with CD (1964–2003) and 64 118 age‐matched and sex‐matched individuals without CD. Cox regression was used to estimate the risk of vascular disease in subjects with CD. Analyses were restricted to individuals with a follow‐up of >1 year and with no vascular disease before study entry.
Results
CD was associated with myocardial infarction (HR 1.27; 95% CI 1.09 to 1.48), angina pectoris (1.46; 1.25 to 1.70), heart failure (1.41; 1.22 to 1.62), brain haemorrhage (1.40; 1.05 to 1.88) and ischaemic stroke (1.35; 1.14 to 1.60). These risk estimates were similar when analyses were restricted to adults in whom vascular disease had been listed as the main diagnosis. In post‐hoc analyses, where reference individuals were restricted to inpatients, no association was found between CD and later vascular disease, except for a lower risk of heart failure (0.79; 0.68 to 0.92).
Conclusions
The positive association between CD and later vascular disease may be explained by ascertainment bias.
Coeliac disease (CD) affects up to 1% of the population in some countries,1,2 and has been linked to a number of complications. Treatment consists of a life‐long gluten‐free diet. Recent data, however, indicate that, despite dietary compliance, mucosal inflammation may persist for many years,3 and even after ten years with a gluten‐free diet some individuals with CD exhibit raised homocysteine levels.4 Homocysteine may be an important risk factor for vascular disease.5,6,7
Research into the causes of death among 10 032 Swedish individuals with CD found an increased risk of both ischaemic heart disease (standardised mortality rate 1.5; 95% CI 1.3 to 1.8) and cerebrovascular disease in study participants (standardised mortality rate 1.4; 95% CI 1.1 to 1.9).8 By contrast, other research has suggested that those individuals with CD9,10 and those positive for antiendomysial autoantibodies11 are at a lower risk of certain vascular disease.10 Despite the decreased risk of hypertension and hypercholesterolaemia in the study by West et al,10 it did not, however, find lower risk estimates for myocardial infarction (MI; hazard ratio (HR) 0.85; 95% CI 0.63 to 1.13) or stroke (HR 1.29; 95% CI 0.98 to 1.70).
The contradictory results of previous research merit further study of CD and cardiovascular disease, especially since inflammatory activity in another immune disorder, rheumatoid arthritis, is linked to an increased risk of vascular disease.12 The objective of this study was to investigate the risk of cardiovascular disease in a cohort of 13 358 individuals with CD and in a comparison cohort of 64 118 subjects from the general population.
Methods
We used the Swedish national Inpatient Register (IPR) to identify all individuals with a diagnosis of CD on discharge from hospital between 1964 and 2003. CD was defined according to the appropriate international classification codes (International Classification of Diseases (ICD); supplementary Appendix is available online at http://heart.bmj.com/supplemental). The IPR was set up in 1964 and has covered all of Sweden since 1987. It contains individual data and is maintained by the Swedish National Board of Health and Welfare. Every record in the IPR can be identified through a personal identity number. The personal identity number is a unique number assigned to more than 99.9% of all Swedish residents and immigrants.13
For every individual with CD, Statistics Sweden identified up to five reference individuals, matched for age, sex, calendar year and residence during the year of CD diagnosis, from the whole population register.14 This register contains information on area of residence, vital status, and dates of immigration or emigration.
We used the following hospital‐based diagnoses (1964–2003) as our end points: MI, angina pectoris (AP), heart failure, brain haemorrhage and ischaemic stroke. We also collected IPR data on diabetes mellitus (DM) and cardiomyopathy in the participants. End points, DM and cardiomyopathy were all defined according to the ICD codes given in the supplementary appendix. The IPR does not distinguish between type 1 and type 2 DM.
Socioeconomic index
In a subset of individuals (n = 43 302; 8436 with CD), we were able to obtain data on socioeconomic index (SEI) from Statistics Sweden (based on occupational classification; table 1).15 Among individuals with data on SEI, some 6500 children were assigned a socioeconomic code on the basis of the occupation of the mother.
Table 1 Characteristics of participants.
| Characteristics | No CD, n (%) (n = 64 118) | CD, n (%) (n = 13 358) |
|---|---|---|
| Age at first‐recorded diagnosis of CD (years) | ||
| 0–15 | — | 9349 (70.0) |
| 16 | — | 4009 (30.0) |
| Sex | ||
| Males | 25 957 (40.5) | 5439 (40.7) |
| Females | 38 161 (59.5) | 7919 (59.3) |
| Calendar period | ||
| 1964–73 | 2368 (3.7) | 490 (3.7) |
| 1974–83 | 18 336 (28.6) | 3789 (28.4) |
| 1984–93 | 28 717 (44.8) | 5950 (44.5) |
| 1994–2003 | 14 697 (22.9) | 3129 (23.4) |
| SEI | ||
| I | 6922 (10.8) | 1446 (10.8) |
| II | 8641 (13.5) | 2018 (15.1) |
| III | 19 303 (30.1) | 4972 (37.2) |
| Missing data | 29 252 (45.6) | 4922 (36.8) |
| Diabetes mellitus* | ||
| Yes | 1262 (2.0) | 769 (5.8) |
| Hypertensive disease† | ||
| Yes | 1253 (2.0) | 238 (1.8) |
| Positive events† | ||
| Myocardial infarction | 1032 (1.6) | 233 (1.7) |
| Angina pectoris | 934 (1.5) | 240 (1.8) |
| Heart failure | 1228 (1.9) | 300 (2.2) |
| Brain haemorrhage | 232 (0.4) | 66 (0.5) |
| Ischaemic stroke | 842 (1.3) | 198 (1.5) |
CD, coeliac disease; SEI, socioeconomic index.
Subjects with >1 year of follow‐up after diagnosis of coeliac disease (CD) or, in matched subjects, the corresponding date.
“I” is the highest category. For reference individuals, we have given the number of individuals who constituted the basis for the Cox regression. We actually had data on SEI in another 5879 reference individuals, but these individuals were not part of the internally stratified calculations, owing to missing values on SEI in the matched individuals with CD. Adding the 5879 reference individuals to those presented above, the proportion of missing values was found to be similar among individuals with CD and among those without CD.
*The inpatient registry does not distinguish between type 1 and type 2 diabetes mellitus.
†Number of positive events before the end of follow‐up. Does not include first year after study entry (and diagnosis of CD).
Exclusion criteria
The Swedish National Board of Health and Welfare identified 15 533 individuals with CD diagnosed before the end of follow‐up. We excluded (1) 94 individuals with CD owing to data irregularities; (2) 1303 individuals with CD and any of the cardiovascular end points before first‐recorded CD diagnosis; and (3) another 778 individuals with CD and <1 year of follow‐up after study entry. Similar exclusion criteria were used for the corresponding age‐matched and sex‐matched reference individuals. The analyses of the current study were therefore based on 13 358 individuals who had received a diagnosis of CD (1964–2003) and 64 118 age‐matched and sex‐matched individuals without CD.
Statistical methods and analyses
Cox regression estimated hazard ratios for cardiovascular disease. The Cox model was internally stratified for sex, age, county and calendar year. The proportional hazard assumption was tested with log minus log plots for each end point. We also plotted Kaplan–Meier survival curves (supplementary appendix). Follow‐up time was started 1 year after study entry (date of first recorded CD diagnosis and corresponding date in matched reference individuals). Follow‐up time ended on the date of first discharge diagnosis of cardiovascular disease, date of emigration, death or the end of the study period (31 December 2003), whichever happened first.
In separate analyses, we stratified for sex and age at study entry (⩽15vs ⩾16 years). Multiplicative interaction terms consisting of sex and CD status versus cardiovascular outcome were inserted when the crude risk estimates for cardiovascular disease indicated a difference according to sex. In separate analyses, we adjusted for DM. We also calculated HRs after excluding all individuals with a diagnosis of DM before the end of follow‐up. In order to increase the specificity of our end points, we calculated the risk of having a diagnosis of vascular disease listed as the main diagnosis. We also tested whether the inclusion of the first year after study entry affected the risk estimates. In a separate analysis of CD and heart failure, we excluded individuals with cardiomyopathy16 to rule out the possibility that an association of CD with heart failure would be due to dilated cardiomyopathy.
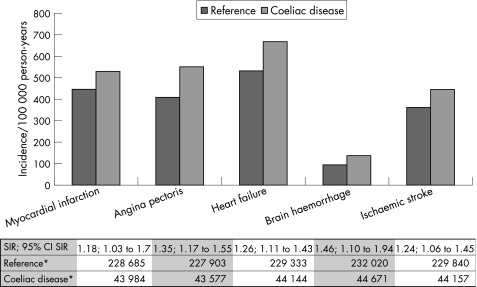
The majority of positive events occurred in individuals entering the study at the age of ⩾16 years. To further investigate the relationship between CD and vascular disease, we calculated standardised incidence ratios for our five end points in this age group (fig 1).
Figure 1 Incidence of vascular disease in individuals entering the study at age 16 years or older. *Person‐years of exposure when the first year after study entry and diagnosis of coeliac disease has been excluded. A person with a diagnosis of myocardial infarction (MI) cannot be exposed to MI after the first MI (but may still be at risk for brain haemorrhage). For that reason, the numbers of person‐years of exposure differ for the various outcome measures. SIR, standardised incidence ratio.
Table 1 gives the number of positive events. In post‐hoc analyses, we estimated the risk of later vascular disease in individuals with CD compared with reference individuals who had been admitted to hospital within <1 year before or after the first diagnosis of CD in the matched individual with CD. We chose to adjust for sex, age and calendar period instead of using internal stratification since some strata consisted of only one individual with CD (and no reference individuals). 95% CIs for HRs not including 1.00 were considered statistically significant. Statistics were calculated using SPSS V.11.0.
Power calculation
At a significance level of 5%, we had an 80% power to detect an increased risk of subsequent MI in individuals with CD if the HR was ⩾1.17. For subsequent ischaemic stroke, we had the power to detect an increased HR of ⩾1.20.
Ethics
This study was approved by the research ethics committee of the Karolinska Institute. None of the participants was contacted. Patient information was anonymised before the analyses.
Results
Characteristics of participants
The median (range) age at study entry (age at first recorded CD diagnosis and the corresponding date in reference individuals) was 2 (0–94) years. The majority of participants were female (table 1). The median (range) age at first recorded MI in individuals with CD was 73 (29–99) years (ischaemic stroke: 73.5 (23–91) years). Low socioeconomic status (SEI III) was more prevalent in individuals with CD (p<0.001).
Main analyses of vascular disease
CD was associated with statistically significant risk increases (HRs within brackets) for MI (1.27), AP (1.46), heart failure (1.41), brain haemorrhage (1.40) and ischaemic stroke (1.35; table 2 and supplementary appendix). Risk estimates were similar when we restricted our end points to vascular diseases listed as main diagnoses (table 2), and when we restricted those analyses to individuals entering the study in adulthood (table 2). The risk estimates were similar after adjustment for DM or when we excluded individuals with DM before the end of follow‐up (table 2). CD diagnosed in childhood was positively associated with later heart failure (HR 3.07; 95% CI 1.00 to 9.39), but not with any other outcome measures of this study (data not shown).
Table 2 Coeliac disease and risk of later vascular disease.
| Outcome | Overall estimates | Adjusted for DM* | Individuals with DM* excluded | Vascular disease is main diagnosis†‡ | Vascular disease is main diagnosis†‡ | Comparison with inpatient reference individuals | First year of follow‐up included |
|---|---|---|---|---|---|---|---|
| HR§, 95% CI | AHR§, 95% CI | HR§, 95% CI | HR§, 95% CI | HR§, 95% CI | HR§, 95% CI | HR§, 95% CI | |
| Myocardial infarction | 1.27, 1.09 to 1.48 | 1.26, 1.08 to 1.47 | 1.23, 1.03 to 1.47 | 1.18, 1.00 to 1.40 | 1.19, 1.00 to 1.41 | 0.93, 0.77 to 1.18 | 1.27, 1.10 to 1.47 |
| Angina pectoris | 1.46, 1.25 to 1.70 | 1.44, 1.23 to 1.68 | 1.37, 1.15 to 1.63 | 1.36, 1.14 to 1.64 | 1.36, 1.41 to 1.64 | 0.98, 0.81 to 1.18 | 1.42, 1.23 to 1.65 |
| Heart failure | 1.41, 1.22 to 1.62 | 1.43, 1.24 to 1.65 | 1.48, 1.26 to 1.74 | 1.45, 1.20 to 1.76 | 1.44, 1.18 to 1.75 | 0.79, 0.68 to 0.92 | 1.56, 1.38 to 1.78 |
| Brain haemorrhage | 1.40, 1.05 to 1.88 | 1.42, 1.06 to 1.90 | 1.34, 0.97 to 1.83 | 1.37, 1.01 to 1.86 | 1.33, 0.96 to 1.83 | 1.26, 0.87 to 1.83 | 1.63, 1.24 to 2.13 |
| Ischaemic stroke | 1.35, 1.14 to 1.60 | 1.34, 1.13 to 1.59 | 1.38, 1.14 to 1.66 | 1.37, 1.14 to 1.64 | 1.38, 1.15 to 1.66 | 0.92, 0.76 to 1.12 | 1.37, 1.17 to 1.60 |
AHR, adjusted hazard ratio; DM, diabetes mellitus.
First year of follow‐up has been excluded in all the above analyses except when otherwise noted.
*DM, type 1 or type 2.
†HR for vascular disease listed as the main diagnosis. All other analyses refer to vascular disease listed as main or secondary diagnosis.
‡Analyses restricted to individuals with coeliac disease diagnosed in adulthood (and reference individuals entering the study in adulthood).
§Estimates derived from Cox's regression.
We had data only on SEI in a subset of individuals. Crude HRs and adjusted HRs were similar for all end points, indicating that SEI is not a major confounder for vascular disease in CD (data not shown). In all, five individuals with CD (two children and three adults) and 18 reference individuals with heart failure had a diagnosis of cardiomyopathy. When we excluded these individuals, the overall HR for heart failure was 1.41 (95% CI 1.22 to 1.62).
Kaplan–Meier plots showed a risk increase for most cases of vascular disease in the first 20–25 years after diagnosis of CD, but not after that (supplementary appendix).
Standardised incidence ratios in adults
The standardised incidence ratios for vascular disease in CD diagnosed in adulthood were similar to the relative risks for vascular disease estimated through Cox regression (fig 1).
Post‐hoc analyses
In post‐hoc analyses, we estimated the risk of vascular disease in 13 358 individuals with CD compared with that in 11 478 reference individuals who had also been hospitalised at the time of study entry. The most common diagnoses underlying initial hospital admission in reference individuals with later MI were DM, cataract of the eye and alcoholism.
In these analyses, we found no association with CD of later vascular disease, except for a lower risk of heart failure (0.79; 0.68 to 0.92; table 2).
Discussion
We found a positive association between CD and MI, AP, heart failure, brain haemorrhage and ischaemic stroke. This risk increase was seen in the first 20–25 years after diagnosis of CD, but not after that. Furthermore, the risk of vascular disease did not differ between individuals with CD and individuals hospitalised for non‐CD reasons.
In contrast with previous studies,9,10,11 we used a matched prospective analysis, internally stratified for a number of factors, including age and sex. This means that our findings are independent of sex, age and calendar year. Female sex is a risk factor in CD,17 whereas male sex is linked to an increased risk of cardiovascular disease.18 Nevertheless, cardiovascular disease is the prime cause of death among women in the Western world.19 We found an increased risk for vascular disease also after taking DM into account. DM is an important risk factor for cardiovascular death, especially in women.20 This is to our knowledge the first study on vascular disease distinguishing between CD diagnosed in childhood and CD diagnosed in adulthood, although it failed to show any association between childhood CD and later vascular disease other than heart failure.
As opposed to earlier research,9,10,11 we excluded individuals with vascular disease before diagnosis of CD or within 1 year after diagnosis of CD. This is important as there is otherwise a risk that ascertainment bias in individuals with CD will contribute to all kinds of morbidity. Still, there remains a risk of ascertainment bias in this study, as cases were identified through hospital discharge diagnoses. In recent years, some patients with stable angina have undergone coronary angiogram as outpatients. However, a low ascertainment of these patients would affect our risk estimates only if the prevalence of coronary angiogram at diagnosis of heart failure differentiates between individuals with and without CD. Currently in Sweden, the perceived need to undergo angiogram is not influenced by the presence of CD. Confirmation of the vascular diagnoses through CT, ultrasound, angiogram and EEG would have been desirable, but is not possible since the Swedish IPR does not contain data on most investigative procedures. Our end points usually entail hospital admission; this is especially so for MI and stroke.
The IPR does not contain data on smoking, body weight or body mass index. Smoking is of major importance to the risk of cardiovascular death.21 Given that smoking and body mass index are negatively associated with CD,11,22,23 inclusion of these variables in our statistical models would probably have increased the risk estimates for vascular disease. Instead, we were able to adjust for SEI (which is indeed strongly linked to smoking habits, other lifestyle factors in Sweden24 and in this study also to CD) in a subset of individuals, and this only affected our risk estimates marginally.
In 1999, Ivarsson et al25 reported a prevalence of diagnosed CD in adults slightly above 1/1000. Since the population in Sweden is about nine million (although a larger number of individuals have been at risk of CD between 1964 and 2003), we assume that a large proportion of Swedish individuals with diagnosed CD have been included in this study, although not all individuals with CD are hospitalised. False‐negative diagnosis in subjects with actual CD is probably uncommon compared with the number of reference individuals without CD, and should not affect the risk estimates.
Individuals identified through a hospital discharge register may suffer from more severe CD than the average individual with CD. However, many individuals were probably hospitalised when undergoing small‐bowel biopsy. This is especially so for small children, but also for adults in the first half of the study period. However, in post‐hoc analyses where reference individuals were restricted to those being hospitalised within 1 year before or after study entry, CD was not associated with an increased risk of later vascular disease. Instead, there was a negative association with CD of later heart failure. Hence, we cannot rule out that the association seen between CD and later vascular disease in the main analyses of this study is due to ascertainment bias and our choice of reference group. Still, it must be remembered that hospitalised‐reference individuals are sicker than the general population. The most common diagnosis at hospital admission in this subset of reference individuals with vascular disease was DM—another autoimmune disease. The identification of individuals with and without CD in this study may explain why our study results differ from those in the West et al's study. Other explanations include different smoking patterns and blood pressure levels in Sweden and England,26 and the larger number of study participants in our study.
Swedish doctors generally perform small intestinal biopsy before confirming the diagnosis of CD.27 This could explain the high specificity for CD found in the IPR; in a study on CD and lymphoma, >85% of patients with an IPR diagnosis of CD and lymphoma had a correct diagnosis of CD.28 Although every study carries a risk of misclassification of end points, this should not be a major drawback of this study. Misclassification only affects risk ratios marginally, provided it is not differential with regard to the exposure variable—in this case CD. We find such misclassification unlikely. Besides, we calculated the risk of vascular disease listed as main diagnosis in order to increase the specificity of our end points. This resulted in similar risk estimates. MI, AP and heart failure were studied in detail through patient charts in a study of some 900 Swedish individuals with ICD‐8 diagnoses.29 For these diagnoses, the specificity was 87–100% (36/36 patients with a main diagnosis of MI actually had MI; 17/18 had AP; and 15/17 had heart failure).29
Suggested mechanism of action
The increased risk of vascular disease seen among patients with CD in this study could be due to ascertainment bias, since we found no positive association between CD and later vascular disease when reference individuals were restricted to inpatients. The relationship between CD and vascular disease may be complicated since CD is linked with protective factors for vascular disease such as lower serum cholesterol levels11 and indications of lower blood pressure,10 but also with risks for vascular disease such as lower folate levels30 and higher homocysteine levels.4 Homocysteine levels in treated CD are only marginally increased,4 but it remains a biologically plausible possibility that low‐grade inflammation31,32 may result in a modest increase in the risk of vascular disease, if our results are not fully explained by ascertainment bias. In rheumatoid arthritis, another disease characterised by inflammation, the risk of death from coronary heart disease is increased by about 70%.12 Although most individuals with CD but on a gluten‐free diet will experience clinical remission, endoscopic abnormalities and histologic inflammation may persist for many years in up to three of four adults reporting diet compliance.3
Conclusion
In conclusion, this study found a positive association between CD and later vascular disease. This may probably be explained by ascertainment bias.
Supplementary Appendix is available online at http://heart.bmj.com/supplemental
Copyright © 2007 BMJ Publishing Group and British Cardiovascular Society.
Abbreviations
AP - angina pectoris
CD - coeliac disease
DM - diabetes mellitus
ICD - International Classification of Diseases
MI - myocardial infarction
IPR - Inpatient Register
SEI - socioeconomic index
Footnotes
Funding: JFL was supported by grants from the Swedish Research Council and the Örebro University Hospital while writing this article. This project was supported by the Swedish Research Council, the Swedish Society of Medicine, the Örebro Society of Medicine, the Majblomman Foundation, the Sven Jerring Foundation, the Karolinska Institutet, The Clas Groschinsky Foundation, the Juhlin Foundation, the Stiftelsen Samariten and the Swedish Coeliac Society. The funding organisations played no role in the design or conduct of the study, in the collection, management, analysis or interpretation of the data, and did not participate in preparation, review or approval of the manuscript.
Competing interests: None.
Supplementary Appendix is available online at http://heart.bmj.com/supplemental
References
- 1.Maki M, Mustalahti K, Kokkonen J.et al Prevalence of celiac disease among children in Finland. N Engl J Med 20033482517–2524. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Berti I, Gerarduzzi T.et al Prevalence of celiac disease in at‐risk and not‐at‐risk groups in the United States: a large multicenter study. Arch Intern Med 2003163286–292. [DOI] [PubMed] [Google Scholar]
- 3.Lee S K, Lo W, Memeo L.et al Duodenal histology in patients with celiac disease after treatment with a gluten‐free diet. Gastrointest Endosc 200357187–191. [DOI] [PubMed] [Google Scholar]
- 4.Hallert C, Grant C, Grehn S.et al Evidence of poor vitamin status in coeliac patients on a gluten‐free diet for 10 years. Aliment Pharmacol Ther 2002161333–1339. [DOI] [PubMed] [Google Scholar]
- 5.Wald D S, Law M, Morris J K. Homocysteine and cardiovascular disease: evidence on causality from a meta‐analysis. BMJ 20023251202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q, Botto L D, Erickson J D.et al Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation 20061131335–1343. [DOI] [PubMed] [Google Scholar]
- 7.Lonn E, Yusuf S, Arnold M J.et al Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 20063541567–1577. [DOI] [PubMed] [Google Scholar]
- 8.Peters U, Askling J, Gridley G.et al Causes of death in patients with celiac disease in a population‐based Swedish cohort. Arch Intern Med 20031631566–1572. [DOI] [PubMed] [Google Scholar]
- 9.Whorwell P J, Alderson M R, Foster K J.et al Death from ischaemic heart‐disease and malignancy in adult patients with coeliac disease. Lancet 19762113–114. [DOI] [PubMed] [Google Scholar]
- 10.West J, Logan R F, Card T R.et al Risk of vascular disease in adults with diagnosed coeliac disease: a population‐based study. Aliment Pharmacol Ther 20042073–79. [DOI] [PubMed] [Google Scholar]
- 11.West J, Logan R F, Hill P G.et al Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 200352960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattar N, McCarey D W, Capell H.et al Explaining how “high‐grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 20031082957–2963. [DOI] [PubMed] [Google Scholar]
- 13.Lunde A S, Lundeborg S, Lettenstrom G S.et al The person‐number systems of Sweden, Norway, Denmark, and Israel. Vital Health Stat 2 198021–59. [PubMed] [Google Scholar]
- 14.Johannesson I.The Total Population Register of Statistics Sweden. New possibilities and better quality. Örebro: Statistics Sweden, 2005
- 15.Guteland G.Socioekonomisk indelning (SEI). (Socioeconomic classification). [in Swedish] Reports on Statistical Co‐ordination. Stockholm: Statistics Sweden (SCB ‐ Statistiska centralbyrån), 19824
- 16.Fonager K, Sorensen H T, Norgard B.et al Cardiomyopathy in Danish patients with coeliac disease. Lancet 19993541561. [DOI] [PubMed] [Google Scholar]
- 17.Ivarsson A, Hernell O, Nystrom L.et al Children born in the summer have increased risk for coeliac disease. J Epidemiol Community Health 20035736–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelsohn M E, Karas R H. Molecular and cellular basis of cardiovascular gender differences. Science 20053081583–1587. [DOI] [PubMed] [Google Scholar]
- 19.American Heart Association and American Stroke Association Heart disease and Stroke Statistics. 2005 Update. Dallas, TX: American Heart Association, 2005, http://www.americanheart.org/presenter.jhtml?identifier = 3000090 (accessed 27 Feb 2007)
- 20.Kannel W B, McGee D L. Diabetes and cardiovascular disease. The Framingham study. JAMA 19792412035–2038. [DOI] [PubMed] [Google Scholar]
- 21.Pocock S J, McCormack V, Gueyffier F.et al A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ 200132375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snook J A, Dwyer L, Lee‐Elliott C.et al Adult coeliac disease and cigarette smoking [see comments]. Gut 19963960–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardella M T, Fredella C, Prampolini L.et al Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten‐free diet. Am J Clin Nutr 200072937–939. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom M, Moghaddassi M, Bolin K.et al Social participation, social capital and daily tobacco smoking: a population‐based multilevel analysis in Malmo, Sweden. Scand J Public Health 200331444–450. [DOI] [PubMed] [Google Scholar]
- 25.Ivarsson A, Persson L A, Juto P.et al High prevalence of undiagnosed coeliac disease in adults: a Swedish population‐based study. J Intern Med 199924563–68. [DOI] [PubMed] [Google Scholar]
- 26.Wolf‐Maier K, Cooper R S, Banegas J R.et al Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 20032892363–2369. [DOI] [PubMed] [Google Scholar]
- 27.Danielsson L, Stenhammar L, Ascher H.et al Proposed criteria for diagnosis of celiac disease in children. Lakartidningen 1998952342–2343. [PubMed] [Google Scholar]
- 28.Smedby K E, Akerman M, Hildebrand H.et al Malignant lymphomas in coeliac disease: evidence of increased risks for lymphoma types other than enteropathy‐type T cell lymphoma. Gut 20055454–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson A C, Spetz C L, Carsjo K.et al Reliability of the hospital registry. The diagnostic data are better than their reputation. Lakartidningen 199491598, 603–598, 605. [PubMed] [Google Scholar]
- 30.Bazzano L A, He J, Ogden L G.et al Dietary intake of folate and risk of stroke in US men and women: NHANES I Epidemiologic Follow‐up Study. National Health and Nutrition Examination Survey. Stroke 2002331183–1188. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes J L, Mamoni R L, Orford J L.et al Increased Th1 activity in patients with coronary artery disease. Cytokine 200426131–137. [DOI] [PubMed] [Google Scholar]
- 32.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 20051113481–3488. [DOI] [PubMed] [Google Scholar]